Reducing Dietary Sodium to Less Than 100 Mmol in Heart Failure (SODIUM-HF): An International, Open-Label, Randomized, Controlled Trial
Background
Dietary sodium restriction has been suggested to prevent fluid overload and adverse outcomes for patients with heart failure.
We designed the Below 100 mmol Dietary Intervention in Heart Failure (SODIUM-HF) Study to test whether or not a reduction in dietary sodium reduces the incidence of future clinical events.
Methods
SODIUM-HF is an international, open-label, randomized, controlled trial that enrolled patients at 26 sites in six countries (Australia, Canada, Chile, Colombia, Mexico and New Zealand). Eligible patients were 18 years or older, with chronic heart failure ( New York Heart Association [NYHA] functional class 2-3), and receiving guideline-directed and optimally tolerated medical treatment.
Patients were randomly assigned (1:1), using a standard number generator and variable block sizes of two, four, or six, stratified by site, to usual care according to local guidelines or a low-sodium diet. of less than 100 mmol (i.e., <1500 mg/day).
The primary outcome was the composite of cardiovascular disease-related hospital admission, cardiovascular disease-related emergency department visit, or death from all causes within 12 months in the intention-to-treat (ITT) population (i.e., all randomized patients). Safety was evaluated in the ITT population.
This study is registered with ClinicalTrials.gov NCT02012179.
Results
Between March 24, 2014 and December 9, 2020, 806 patients were randomly assigned to a low-sodium diet (n=397) or usual care (n=409).
The median age was 67 years (IQR 58-74) and 268 (33%) were women and 538 (66%) were men.
Between baseline and 12 months, median sodium intake decreased from 2286 mg/day (IQR 1653–3005) to 1658 mg/day (1301–2189) in the low-sodium group and from 2119 mg/day (1673– 2804) to 2073 mg/day (1541-2900) in the usual care group.
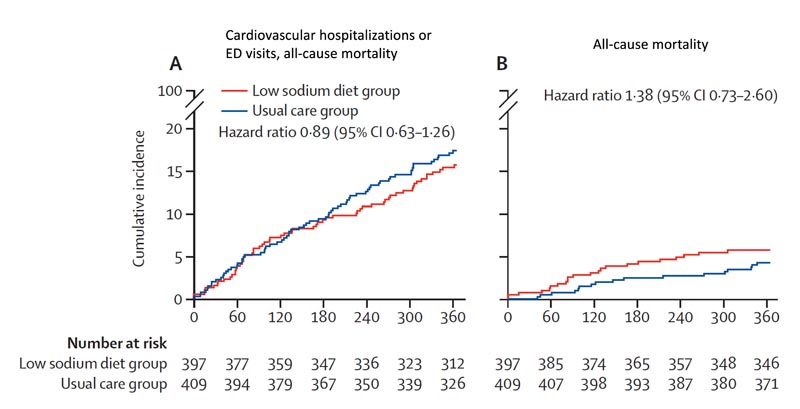
At 12 months , events comprising the primary outcome had occurred in 60 (15%) of 397 patients in the low-sodium diet group and 70 (17%) of 409 in the usual care group ( hazard ratio [HR ] 0·89 [95% CI 0·63–1·26], p=0·53).
Death from all causes occurred in 22 (6%) patients in the low-sodium diet group and 17 (4%) in the usual care group (HR 1·38 [0·73–2·60]; p =0·32), cardiovascular-related hospitalization occurred in 40 (10%) patients in the low-sodium diet group and 51 (12%) patients in the usual care group (HR 0·82 [0·54 –1·24]; p=0·36), and cardiovascular disease-related emergency department visits occurred in 17 (4%) patients in the low-sodium diet group and 15 (4%) patients in the low-sodium diet group. usual care (HR 1·21 [0·60–2·41];p=0·60).
No safety events related to study treatment were reported in either group. Cardiovascular-related hospitalization occurred in 40 (10%) patients in the low-sodium diet group and 51 (12%) patients in the usual care group (HR 0.82 [0.54–1.24]; p = 0.36), and cardiovascular disease-related emergency department visits occurred in 17 (4%) patients in the low-sodium diet group and 15 (4%) patients in the usual care group (HR 1 ·21 [0·60–2·41]; p=0·60).
No safety events related to study treatment were reported in either group. Cardiovascular-related hospitalization occurred in 40 (10%) patients in the low-sodium diet group and 51 (12%) patients in the usual care group (HR 0.82 [0.54–1.24]; p = 0.36), and cardiovascular disease-related emergency department visits occurred in 17 (4%) patients in the low-sodium diet group and 15 (4%) patients in the usual care group (HR 1 ·21 [0·60–2·41]; p=0·60). No safety events related to study treatment were reported in either group.
Interpretation
In outpatients with heart failure, a dietary intervention to reduce sodium intake did not reduce clinical events .
Research in context
Added value of this study
To our knowledge, our study is the largest randomized clinical trial to date to test a dietary sodium reduction strategy for patients with heart failure. We found that reducing dietary sodium (to a goal of <1500 mg/day) in patients with heart failure did not reduce the composite clinical outcome of all-cause mortality, cardiovascular-related hospitalization, or related emergency department visits. with cardiovascular diseases compared to usual care for 12 months.
Improvement was seen in patient-reported quality of life outcome and New York Heart Association physician-assessed functional class ; however, no significant differences were observed between groups in 6-minute walk distance.
Therefore, our study provides high-quality evidence to guide clinical decision-making in a field that has so far lacked long-term results,
Implications of all available evidence
Because the degree of dietary sodium reduction that would lead to a reduction in clinical events has not yet been defined, clinicians and patients should consider a dietary intervention similar to other medical therapies and balance the potential benefits on an individual basis. .
References:
Ezekowitz JA, Colin-Ramirez E, Ross H, et al., on behalf of the SODIUM-HF Investigators. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomized, controlled trial . Lancet 2022; April 2 / 2022
















