Highlights
|
Background:
Oral and intravenous flecainide is recommended for cardioversion of atrial fibrillation. In this open-label dose escalation study, the feasibility of administering flecainide by oral inhalation (flecainide acetate inhalation solution) for acute conversion was evaluated.
We hypothesized that flecainide administered by oral inhalation would rapidly reach plasma concentrations sufficient to restore sinus rhythm in patients with new-onset atrial fibrillation.
Methods:
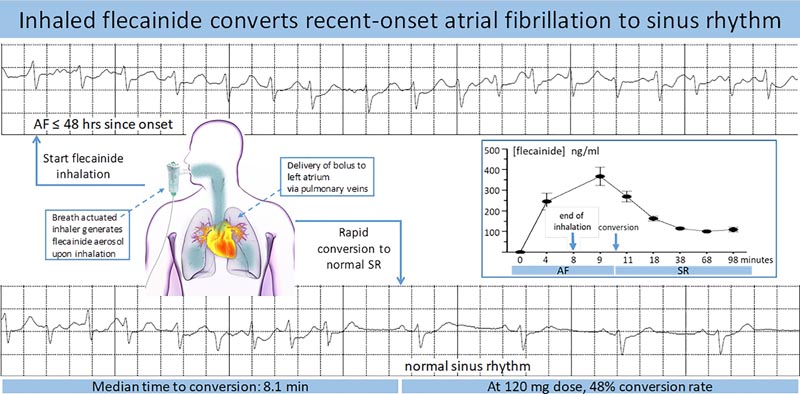
Patients (n=101) with symptomatic atrial fibrillation (for ≤48 hours) flecainide acetate inhalation solution self-administered using a nebulizer (30 mg [n=10], 60 mg [n=22], 90 mg [n=21 ], 120 mg [n=19] and 120 mg in a formulation containing saccharin [n=29]).
Electrocardiograms and plasma flecainide concentrations were obtained, heart rate was monitored by 4-hour Holter monitoring, and adverse events were recorded.
Results:
Conversion rates increased with dose and with peak plasma concentrations of flecainide.
- At the highest dose, 48% of patients transitioned to sinus rhythm within 90 minutes of starting inhalation.
- Among patients who achieved a peak plasma concentration >200 ng/mL, the conversion rate within 90 minutes was 50%; for those who achieved a maximum plasma concentration <200 ng/mL, it was 24%.
- Conversion was rapid (median time to conversion 8.1 minutes from end of inhalation) and conversion led to resolution of symptoms in 86% of responders.
Adverse events were typically mild and transient and included: cough, sore throat, throat irritation; at the highest dose with the saccharin-containing formulation, these adverse events were reported by 41%, 14%, and 3% of patients, respectively.
Cardiac adverse events consistent with those seen with oral and intravenous flecainide were rare and included postconversion pauses (n=2), bradycardia (n=1), and atrial flutter with 1:1 atrioventricular conduction (n=1); none required treatment and all resolved without sequelae.
Conclusions:
Administration of flecainide by oral inhalation has been shown to be safe and produces plasma concentrations of flecainide sufficient to restore sinus rhythm in patients with new-onset atrial fibrillation.
What is known?
- Intravenous or oral flecainide can rapidly restore normal sinus rhythm in patients with new-onset atrial fibrillation.
- The limitation of intravenous flecainide includes the requirement for in-hospital administration of the drug.
- The limitation of oral flecainide is the relatively slow onset of action.
What the study adds
Oral inhalation flecainide can quickly and safely convert a significant proportion of patients with new-onset atrial fibrillation to normal sinus rhythm.
This new mode of administration may become a practical option for rapid conversion of atrial fibrillation with rapid relief of symptoms, shorter stays in an emergency department, and reduced hospital admissions.
Conclusions
The results of this study show for the first time that it is feasible to administer flecainide by oral inhalation for the conversion of new-onset AF to SR, achieving a conversion rate of up to 50% within 90 minutes after initiation of inhalation. The conversion rate depended on the dose, inhalation solution and the Cmax achieved.
Overall, administration of flecainide by oral inhalation was safe and no major complications were reported.
Therefore, the results of this preliminary evaluation of administering flecainide via oral inhalation for cardioversion of new-onset symptomatic AF suggest that orally inhaled flecainide has the potential to be a practical option for conversion. rapid transition from AF to RS and immediate relief of symptoms, resulting in shorter stays in an emergency department and therefore reduced hospital admissions.
In the future, if the safety profile justifies it, this method of flecainide administration may be developed for out-of-hospital use, similar to the pill in the pocket,18 but allowing for faster restoration of SR.















