Trigeminal neuralgia, traditionally called tic douloureux , is a chronic neuropathic pain disorder characterized by spontaneous and provoked paroxysms of stabbing or electric shock-like pain in a region of the face. Poor quality of life and suicide in severe cases have been attributed to the disorder.
Clinical characteristics and diagnosis
The diagnosis of trigeminal neuralgia is clinical and is based on three main criteria:
- Pain restricted to the territory of one or more divisions of the trigeminal nerve.
- Paroxysms of pain that are sudden, intense, and very brief (less than 1 second to 2 minutes, but usually a few seconds) and are described as a "shock" or an "electric sensation."
- Pain triggered by innocuous stimuli on the face or intraoral trigeminal territory.
Paroxysmal triggered pain is particular to trigeminal neuralgia and is reported by 91 to 99% of patients, indicating that this feature may be pathognomonic of trigeminal neuralgia.
Trigeminal neuralgia pain most often affects the distribution of the second (maxillary) or third (mandibular) division of the trigeminal nerve, affecting the right side of the face more often than the left side.
Bilateral trigeminal neuralgia is rare and should raise concern for the possibility of facial neuralgia due to an underlying neurological disease or a non-neurological disorder affecting the skull.
The incidence of trigeminal neuralgia is higher among women than men and increases with age.
Many forms of facial pain have been combined with trigeminal neuralgia, but they are likely to be distinct entities, sometimes included in the category of "atypical facial pain" or "painful trigeminal neuropathy . "
The posterior third of the scalp, the external ear (except for the tragus), and the skin overlying the angle of the jaw are not innervated by the trigeminal nerve and are not sites of pain due to trigeminal neuralgia (see figure below ); pain in these areas suggests a different process.
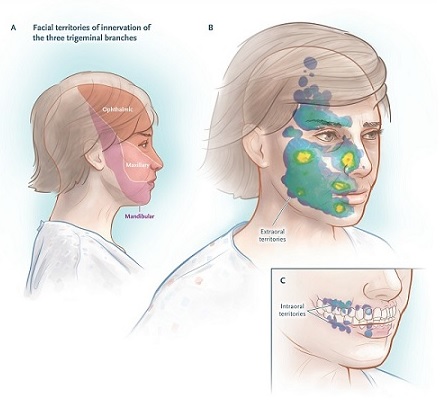
Territories of innervation of the trigeminal nerve and distribution of the activation zone.
The pain of trigeminal neuralgia can be triggered by common gestures of daily life, and the triggers are found in small receptive sensory zones, for example, the touch of a napkin or tissue on the upper lip or even a breeze flowing through of a sensitive area of the face.
The location of the pain is not always consistent with the location of the sensory trigger. For example, stimuli in and around the lower lip may induce pain in the temple, or sensory triggers in the lateral portions of the nose may induce shock-like pain that radiates to the forehead or upper lip.
The specific activation maneuvers of a series of patients are shown in the following table, and the distribution of the activation zones that cause pain is shown in the figure above. Few patients report no triggers.
Examination of trigeminal neuralgia includes observation of the face while the patient sits completely still. With a spontaneous paroxysm of trigeminal neuralgia, the doctor may notice blinking or a small movement of the mouth that is unknown to the patient.
Less commonly, during a paroxysmal attack, a forceful contraction of the facial muscles, called a “tic convulsive,” may occur. Sensory examination of the face is generally unrevealing in cases of trigeminal neuralgia, although some patients report areas of mild hypoesthesia.
Types and causes
Three types of trigeminal neuralgia have been delineated: classic, secondary and idiopathic. The classic type , which is the most common, is caused by intracranial vascular compression of the trigeminal nerve root , as described below. The responsible vessel is usually the superior cerebellar artery , which induces morphological changes in the adjacent trigeminal nerve root.
Secondary trigeminal neuralgia , which represents approximately 15% of cases, is attributable to an identifiable neurological disease such as multiple sclerosis or a tumor in the cerebellopontine angle, which alters the entry zone of the trigeminal nerve root or compresses the nerve in its extracranial part.
Idiopathic trigeminal neuralgia , in which no apparent cause can be found, accounts for approximately 10% of cases.
The clinical features of classic and secondary trigeminal neuralgia are similar, although patients with secondary trigeminal neuralgia tend to be younger , more likely to have sensory loss in one part of the face, and more likely to have bilateral pain .
Because the three forms of trigeminal neuralgia may be clinically indistinguishable, it is advisable to perform gadolinium magnetic resonance imaging (MRI) to rule out multiple sclerosis and cerebellopontine masses at the time of initial diagnosis.
A recent study showed rare variants in genes encoding voltage-gated ion channels in patients with a family history of classic or idiopathic trigeminal neuralgia, but the frequency and clinical significance of this finding is unknown.
Neurovascular compression in classic trigeminal neuralgia
Over the past few decades, the classic form of trigeminal neuralgia has been revealed through the work of Jannetta et al, and the potential for cure by intracranial microvascular surgery has been studied.
The pathophysiology is considered to be compression of the sensory portion of the trigeminal nerve , near its entry area into the pons, by a small adjacent branch of the basilar artery, most often the superior cerebellar artery . However, simple contact between the nerve and a vascular structure does not appear to be adequate to cause or explain the disorder.
To attribute the disorder to neurovascular compression , the ideal would be to demonstrate that the anomalous vessel induces anatomical alterations in the trigeminal root, such as distortion or atrophy. The most characteristic finding during the operation is a small tortuous artery or arterial loop that impinges on the medial aspect of the trigeminal root in its entry area, causing lateral dislocation, distortion, flattening or atrophy of the nerve root.
Neurovascular compression can be seen with the use of MRI and three-dimensional reconstruction. Imaging techniques include three-dimensional T2 MRI sequences with detailed examination of the cisternal and cavernous segments of the nerve, three-dimensional magnetic resonance angiography for visualization of arteries, and phase-contrast MRI to show veins.
Vascular decompression can reverse these abnormalities in the trigeminal root entry zone, where the sensory portion of the nerve enters the pons.
Pathophysiology
At its entrance to the pons, the trigeminal nerve (like all peripheral nerves) loses its myelin sheath from Schwann cells and is replaced by central myelin generated by oligodendroglia.
This transition zone is vulnerable to damage and particularly demyelination . Vascular compression is the usual cause of demyelination before the nerve enters the pons, and multiple sclerosis is the typical cause just after entry into the pons. Demyelination at these sites has been demonstrated in neurophysiological, neuroimaging, and histological studies.
When the myelin sheath becomes thin enough to allow transmembrane passage of ions into the underlying axon, the axon is not equipped to pump sodium rapidly.
The resulting depolarization makes the axon hyperexcitable , causing ectopic generation of impulses with high-frequency afterdischarges (discharges that occur after termination of the stimulus) and crosstalk between fibers (called ephaptic transmission). Histological evidence indicates that the nerve fibers most involved in demyelination are A-β fibers (large, non-nociceptive fibers), which are the most susceptible to demyelination from mechanical damage or multiple sclerosis.
It has been proposed that high-frequency discharges originating at the site of demyelination along A-β primary afferents are redirected by brainstem neurons to be perceived as paroxysmal pain .
Some researchers have observed excessive excitability or reductions in the volume of several cortical and subcortical brain areas in patients with trigeminal neuralgia, but these changes are probably a consequence of adaptation to chronic stimulation of these regions.
Trigeminal neuralgia with continuous pain
Although paroxysmal facial pain is the hallmark of trigeminal neuralgia, 24 to 49% of patients report continuous or prolonged pain between paroxysmal attacks.
Fluctuating background pain , with a distribution that is consistent with that of paroxysmal pain, is described as burning, throbbing pain, or aching . Trigeminal neuralgia characterized by this symptom, regardless of the cause, has previously been classified as type 2 or atypical trigeminal neuralgia and is now classified as trigeminal neuralgia with concomitant continuous pain .
The mechanism underlying continuous pain is different from the mechanism underlying paroxysmal pain, as suggested by the lesser degree of relief of continuous pain, compared with paroxysmal pain, after treatment with sodium channel blockers or microvascular decompression.
The pathophysiological link between the two pain entities is uncertain. Progressive nerve root damage and central sensitization mechanisms have been hypothesized. Burning, stabbing, or aching pain is likely mediated by impairment of C fibers (unmyelinated sensory axons that transmit impulses slowly), as shown in other neuropathic pain conditions.
Loss of C fibers in the trigeminal sensory root can cause abnormal spontaneous activity in second-order neurons in the brain stem. The previous notion that ongoing pain develops as a result of long-standing trigeminal neuralgia is not supported by more recent data.
Secondary trigeminal neuralgia
In 15% of patients with typical pain attacks, trigeminal neuralgia is caused by multiple sclerosis or benign tumors in the cerebellopontine angle. The risk of trigeminal neuralgia is increased 20-fold among patients with multiple sclerosis compared to the general population, with a prevalence of 2 to 5% among patients with multiple sclerosis.
Trigeminal neuralgia sometimes manifests as a clinically isolated syndrome in patients with multiple sclerosis; The age of onset of multiple sclerosis is higher in these patients than in those who do not have trigeminal neuralgia.
A neuroimaging study has shown an association between neurovascular compression and trigeminal neuralgia related to multiple sclerosis, suggesting that they may coexist and be additive. The frequency of this dual mechanism is unknown, but it has implications for treatment.
Pharmacological treatment of trigeminal neuralgia pain in patients with multiple sclerosis is challenging due to side effects of the medications, worsening of multiple sclerosis symptoms such as fatigue and ataxia, and limited evidence of their effectiveness in such patients.
Case series indicate that surgical procedures to reduce vascular compression tend to be less effective than in patients with classic trigeminal neuralgia.
Tumors in the cerebellopontine angle that compress the trigeminal nerve root and cause trigeminal neuralgia include acoustic neuromas, meningiomas, epidermoid cysts, and cholesteatomas. Interestingly, trigeminal neuromas (which are rare) have not been associated with trigeminal neuralgia.
In an analysis of data from four studies that included 243 patients with trigeminal neuralgia, tumors were the cause in 20 patients (8%). Compression of the trigeminal nerve by tumors induces focal demyelination of the trigeminal nerve root, triggering the same generation of high-frequency discharges in naked axons that occurs in vascular compression of the nerve. Infiltrating malignant tumors can also cause axonal degeneration, resulting in hypoesthesia in regions of the face and persistent pain.
Trigeminal neuropathies due to trauma and rheumatologic diseases such as systemic lupus erythematosus and scleroderma can manifest as paroxysmal pain mimicking trigeminal neuralgia, but these associations are rare.
Facial trauma, dental procedures, or maxillofacial surgery can damage branches of the trigeminal nerve, causing paroxysmal stinging, similar to an electric shock or burning pain. However, pain attacks last longer than trigeminal neuralgia paroxysms, and most patients also describe continuous severe pain without sensory trigger zones.
Isolated idiopathic trigeminal neuropathy, a benign, bilateral, symmetrical, purely sensory neuropathy, and facial-onset sensory motor neuropathy, a more severe progressive disease, may also initially manifest as unilateral paroxysmal facial pain.
Corneal reflex testing has been used as a neurophysiological technique to detect trigeminal nerve damage.
This diagnostic test is useful in patients who cannot undergo MRI or to detect demyelination and neuropathies that mimic trigeminal neuralgia.
Treatment
Medical treatment
The anticonvulsant agents carbamazepine , at doses of approximately 200 to 1,200 mg per day, and oxcarbazepine (300 to 1,800 mg per day) have been considered the first-line treatments for the control of paroxysmal pain in patients with trigeminal neuralgia, regardless of the cause, with significant pain control in almost 90% of patients.
The treatment effect is proposed to be related to the blockade of voltage-gated sodium channels, resulting in the stabilization of hyperexcited neuronal membranes and the inhibition of repetitive firing.
However, clinical improvement is often accompanied by side effects, including dizziness, diplopia, ataxia, and elevated aminotransferase levels, which may lead to treatment discontinuation in 23% of patients.
Oxcarbazepine may have fewer side effects than carbamazepine, although it may be discontinued due to excessive central nervous system depression or dose-related hyponatremia.
Contraindications to the use of sodium channel blockers include cardiac conduction problems and allergic reactions, with a high degree of cross-reactivity (40 to 80%) with aromatic antiepileptic drugs.
Carbamazepine and oxcarbazepine reduce the high-frequency discharges that characterize shock-like paroxysms, but the effect of these drugs on the concomitant continuous pain is usually limited.
Gabapentin, pregabalin, and antidepressant agents, which have been shown to be effective in the treatment of other neuropathic conditions characterized by ongoing pain, may be tried as additional agents along with oxcarbazepine or carbamazepine.
Clinical experience suggests that gabapentin may have a lesser effect on trigeminal neuralgia than carbamazepine and oxcarbazepine, but it is associated with a lower incidence of adverse events and may be attempted as monotherapy or as adjunctive therapy, even in patients with multiple sclerosis. .
If medical treatment is ineffective or associated with unacceptable side effects, surgical decompression of the trigeminal nerve may be considered.
Local surgical procedures
Although surgical procedures are effective in reducing the severity and frequency of trigeminal neuralgia attacks in appropriately chosen patients, this type of surgery is usually performed only if standard doses of medications are not sufficient to control symptoms or if the effects side effects prevent its continued use.
A group of surgical interventions, now used infrequently, involve peripheral blockade of the branches of the trigeminal nerve as they emerge from the facial bones by neurectomy, alcohol injections, or induction of radiofrequency lesions or cryolesions.
The purpose of these procedures is to produce an area of anesthesia on the face that corresponds to the distribution of the damaged nerve. However, the benefit of such treatments has not been adequately supported by trials, and the procedures often led to painful anesthesia (severe pain in the area of sensory loss).
A second group of interventions aims to percutaneously damage the trigeminal ganglion in Meckel’s cavum or the exit of the branches of the ganglion at the base of the skull by means of radiofrequency thermocoagulation, chemical destruction through the injection of glycerol, or compression mechanics through balloon inflation.
Radiofrequency thermocoagulation preferentially damages small diameter painful fibers. To prevent corneal deafferentation and resulting keratitis, the electrode is oriented to avoid damaging the first division of the trigeminal nerve. Balloon compression and glycerol injection preferentially damage large myelinated fibers.
Pain relief is immediate with these techniques. Trigeminal sensory deficits are usually transient with balloon compression and glycerol injection and are more severe and long-lasting after radiofrequency thermocoagulation.
Generation of a trigeminal root lesion with a gamma knife is a more recently introduced procedure and is supported by several studies. In contrast to the immediate pain relief associated with percutaneously caused trigeminal ganglion lesions, the analgesic effect of stereotactic gamma knife radiosurgery takes 6 to 8 weeks to develop. Approximately 24 to 71% of patients report continued pain relief 1 to 2 years after undergoing the procedure, and 33 to 56% report continued pain relief at 4 to 5 years.
Facial numbness has been reported in 16% of patients, while painful anesthesia is virtually absent. A meta-analysis showed that approximately 34% of patients do not experience pain relief at one year and require repeat procedures.
Microvascular decompression
Microvascular decompression has now become the surgical procedure of choice for the majority of cases of trigeminal neuralgia that do not respond to medication.
The neurosurgeon identifies the vessel that is compressing the trigeminal nerve root, moves it from below the nerve to above the nerve if necessary (see figure below), and usually inserts a small sponge to keep the pulsating artery separate from the nerve root.
In approximately 11% of patients, the surgeon finds no neurovascular compression or minimal contact, with no apparent nerve compression. In these cases, the surgeon usually inserts the separator sponge anyway, although the failure rate is higher than when a nerve root distortion is identified. This issue underscores the advantage of using established MRI criteria to identify morphologic changes in the trigeminal root.
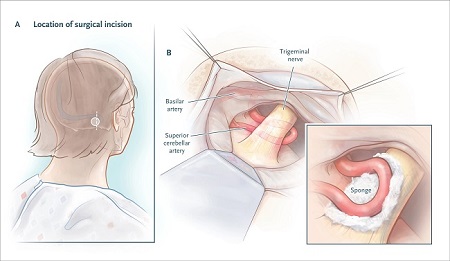
Microvascular decompression.
Meta-analyses have suggested that microvascular decompression is the most effective surgical intervention for classic trigeminal neuralgia. 1 to 2 years after undergoing the procedure, 68 to 88% of patients experience pain relief and 61 to 80% have pain relief at 4 to 5 years.
The average mortality associated with surgery is 0.3%. Cerebrospinal fluid leaks occur in 2.0% of patients, brain stem infarctions or hematomas in 0.6%, and meningitis in 0.4%.
Sensory loss in part or all of the sensory distribution of the trigeminal nerve in the face occurs in 2.9% of patients. The most worrying long-term complication, although rare (incidence, 1.8%), is ipsilateral hearing loss.
Patients with multiple sclerosis who have drug-resistant trigeminal neuralgia may be offered microvascular decompression, although the evidence is insufficient. However, both percutaneous lesions and gamma knife lesions have also been reported to have good outcomes in patients with multiple sclerosis.
Conclusions
|















