Summary Early childhood caries is a serious oral disease that causes aggressive tooth decay. In particular, a synergistic association between a fungus, Candida albicans, and a cariogenic bacteria, Streptococcus mutans, promotes the development of very acidic biofilms that are difficult to eliminate, aggravating virulent damage. These interactions are largely mediated by glycosyltransferases (GtfB) that bind mannans in the C. albicans cell wall. Here, we present an enzymatic approach to target GtfB-mannan interactions in this cross-kingdom consortium using mannan-degrading exo- and endoenzymes. These exo- and endoenzymes are highly effective in reducing biofilm biomass without killing microorganisms, as well as alleviating the production of an acidic pH environment that leads to dental caries. To corroborate these results, we present biophysical evidence using single-molecule atomic force microscopy, biofilm shearing, and enamel surface topography analysis. The data show a drastic decrease in the binding forces of GtfB to C. albicans (approximately 15-fold reduction) after enzymatic treatment. Furthermore, the enzymatic activity disrupted the mechanical stability of the biofilm and significantly reduced the demineralization of human tooth enamel without cytotoxic effects on gingival keratinocytes. Our results represent significant progress toward a novel non-biocidal therapeutic intervention against pathogenic bacterial and fungal biofilms targeting cross-kingdom ligand-receptor binding interactions. Importance Biofilm formation is a key virulence factor responsible for various infectious diseases. In particular, interactions between a fungus, Candida albicans, and a bacteria, Streptococcus mutans, are known to play an important role in the pathogenesis of dental caries. Although some antimicrobials have been applied to treat diseases associated with fungal-involved biofilms, these often lack targeted polymicrobial interactions. Furthermore, these may not be appropriate for preventive measures because these antimicrobials may alter the ecological microbiota and/or induce the prevalence of drug resistance over time. By specifically targeting the interaction mechanism by which mannoproteins on the surface of C. albicans mediate cross-kingdom interaction, we demonstrate that enzymes that degrade mannoproteins can effectively disrupt biofilm interactions without microbicidal effects or without causing cytotoxicity in the cells. human cells. This suggests potential application as a targeted approach to intervene in a cross-kingdom pathogenic biofilm associated with a costly and unresolved oral disease. |
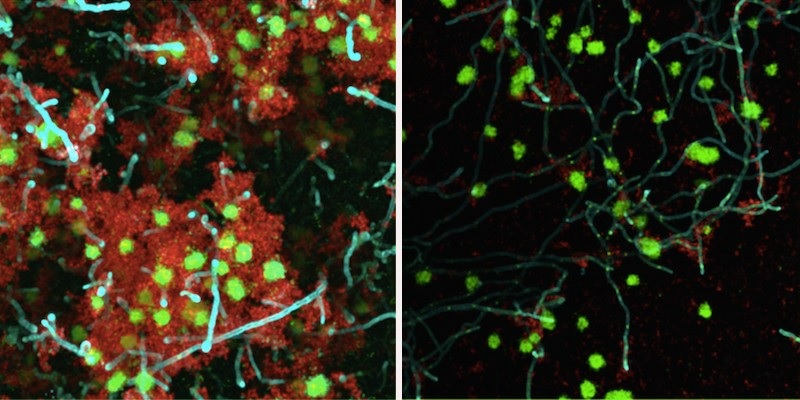
An enzymatic treatment significantly weakened a bacteria-yeast biofilm on a tooth-like surface. This therapeutic approach, which uses beta-mannanase (right panel) to break the links between bacteria and yeast, could be used to treat early childhood caries, a severe form of tooth decay. (Image: Courtesy of Geelsu Hwang)
Comments
By targeting links between bacteria and yeast that can form dental plaque, a new therapeutic strategy could help eliminate the buildup without affecting oral tissues, according to a new study by a team at the University of Pennsylvania.
The combination of a high-carbohydrate diet and poor oral hygiene can leave children with early childhood caries (ECC), a serious form of tooth decay that can have a lasting impact on their oral and overall health.
A few years ago, scientists at Penn’s School of Dental Medicine discovered that the dental plaque that gives rise to CPB is composed of both a bacterial species, Streptococcus mutans , and a fungus, Candida albicans . The two form a sticky symbiosis, known scientifically as a biofilm, which becomes extremely virulent and difficult to dislodge from the tooth surface.
Now, a new study from the group offers a strategy to disrupt this biofilm by targeting the yeast-bacteria interactions that make ECC plaques so untreatable. Unlike some current treatments for CPB, which use antimicrobial agents that can have unwanted effects and potentially damage healthy tissues, this treatment uses an enzyme specific to the bonds that exist between microbes.
"We thought this could be a new way to address the CEC problem that would intervene in the synergistic interaction between bacteria and yeast," says Geelsu Hwang, assistant professor at Penn Dental Medicine and senior author of the study, published in the journal. mBio. "This gives us another tool to disrupt this virulent biofilm."
The work builds on findings from a 2017 paper by Hwang and colleagues, including Hyun (Michel) Koo of Penn Dental Medicine, who found that molecules called mannans in the cell wall of Candida bind tightly to an enzyme secreted by S. mutans, glycosyltransferases (Gftb). ). In addition to facilitating cross-kingdom binding, Gftb also contributes to the stubbornness of dental biofilms by manufacturing glue-like polymers called glucans in the presence of sugars.
While some cases of ECC are treated with medications that kill the microbes directly, potentially reducing the number of pathogens in the mouth, this does not always effectively break down the biofilm and can have unwanted effects on "good" microbes as well as the soft tissues of the oral cavity.
Hwang and his colleagues wanted to try a different approach that directly targeted the insidious interaction between yeast and bacteria and chose to target mannans on the surface of the Candida cell as a contact point.
Using three different mannan- degrading enzymes , they applied each to a biofilm growing on a tooth-like surface in human saliva medium and left it for five minutes. After treatment, they noticed that the total volume of the biofilm was reduced. Using powerful microscopy, they also observed dramatic reductions in biofilm thickness and interactions between bacteria and yeast. The pH of the surrounding medium was higher when exposed to the enzymes, indicating an environment that is not as acidic and therefore less conducive to tooth decay.
They also measured how easy it was to break down the biofilm after treatment, using a device that applies tension, similar to brushing your teeth.
"The biofilm structure was more fragile after enzyme treatment," says Hwang. "We could see that the biofilms were removed more easily."
To confirm the mechanism of their approach, that mannan-degrading enzymes were weakening the bond between yeast and bacteria, the team used atomic force microscopy to measure the bonds between Candida and Gftb. They found that the therapy reduced this bond strength by 15-fold.
Finally, they wanted to get an idea of how well tolerated these enzymes would be when used in the oral cavity, especially since children would be the target patient group.
When applying the enzymes to human gingival cells in culture, they found no harmful impact, even when they used a concentrated form of the enzymes. Additionally, they observed that the treatment did not kill bacteria or yeast, a sign that it could work even if the microbes developed mutations that would give them resistance against other types of therapies.
The researchers kept the application time relatively short at five minutes, although they expect to see activity in an even shorter time, like the two minutes recommended for brushing teeth. Hwang says they can consider an alcohol-free mouthwash with these added enzymes that children could use as a preventative measure against CHD.
The researchers hope to continue pursuing this possibility with additional follow-up, including testing these enzymes in an animal model. With further successes, they aim to add another tool to combat the public health threat of CHD.
















