T cell responses likely play an important role in the control of SARS-CoV-2 infection, but have been relatively little studied. Data now suggest that most infected people develop robust and long-lasting T cell immunity, which has implications for the durability of immunity and future vaccine approaches.
SARS-CoV-2 infections result in highly heterogeneous clinical outcomes, ranging from no symptoms to severe illness and death. Given the suitability of this virus in humans thus far and its likely persistence in this reservoir, it is important to understand the quality and durability of the immunological memory that is produced by the infection.
Although several studies have reported that individuals develop robust SARS-CoV-2-specific memory T cell responses following natural infection, it is unclear whether these responses correlate with clinical or immunological outcomes. In the current issue of Nature Immunology, Zuo et al.6 characterize the durability and diversity of memory T cell responses established after asymptomatic or mild COVID-19.
The authors studied T cell responses six months after infection in 100 people (median age 41 years) who had relatively mild infections (56 people) or asymptomatic infections (44 people). To enumerate T cells that recognized SARS-CoV-2, cells from previously infected individuals were first stimulated with peptides from SARS-CoV-2 proteins to elicit a cytokine response. SARS-CoV-2-reactive T cells were then counted based on secretion of the proinflammatory cytokine interferon (IFN)-γ in an ELISpot (enzyme-linked immunosorbent stain) assay. Almost all donors had a reactive T cell response to SARS-CoV-2 in this trial.
However, the magnitude of the responses was highly variable within the cohort, and a correlate of the response was the presence of symptoms at the onset of infection. People with symptomatic SARS-CoV-2 infections had IFN-γ-producing T cell responses of significantly greater magnitude at six months post-infection compared to those with asymptomatic infection. Although people with severe disease were not included in this study, another recent report found no significant differences in the magnitude of SARS-CoV-2-specific T cell responses between participants who were hospitalized and those who were not.
Importantly, the responses of IFN-γ-producing T cells did not correlate with the age of the subjects within the cohort. Together with a separate report that found that T cell responses in COVID-19 patients increased with age7, the data are reassuring that SARS-CoV-2 infection can elicit robust T cell responses regardless of the age.
The quality of the T cell response was then characterized at greater granularity by quantifying virus-specific CD4 + and CD8 + T cell subsets and intracellular cytokine production (IFN-γ, interleukin (IL)-2, IL -4 and tumor necrosis factor) by these cells. SARS-CoV-2-specific CD4+ T cells were approximately twice as abundant as CD8+ T cells, and cytokine production differed between these populations; for example, more IL-2 was produced in CD4 + T cells, but more IFN-γ was produced in CD8 + T cells.
The greater CD4 + versus CD8 + T cell response is consistent with the findings of a separate report5, which analyzed 43 patients 6 to 8 months after infection, and is independent of age, sex, or whether the initial infection was symptomatic . Interestingly, IL-2, with or without IFN-γ, was the dominant CD4+ cytokine produced in response to both spike (S) and non-spike protein stimulation, and this was confirmed by ELISpot cell culture supernatant analysis. .
Zuo et al. We next studied whether the magnitude of the T cell IFN-γ response six months after infection correlated with the antibody response over time. An interesting feature of this study was the monthly antibody characterization after diagnosis. This allowed a kinetic analysis of IgG levels specific for the SARS-CoV-2 S protein and nucleoprotein (N). These levels were highly heterogeneous among study participants.
On average, IgG responses began to decline after approximately two months, but remained well above the limit of detection in most subjects at five months.
Interestingly, a greater magnitude of the S-specific T cell response at six months was correlated with higher peak antibody levels against the S and N proteins and a sustained antibody response against the N protein. It remains to be determined whether the T cell response is associated with other characteristics of the antibody (e.g., neutralizing capacity and/or other effector functions) and/or whether other characteristics of the T cell response (e.g., production of IL by cells T CD4 +) -2) are also associated with maximal antibody response.
While T cell response was assessed in this study six months after infection, it is unclear whether any participants had been reinfected during that time. This is unlikely, given the low rate of reinfections within six months of a primary SARS-CoV-2 infection, but this could have influenced measurements in some participants.
Furthermore, although the authors evaluated the T cell response relative to the earlier antibody response, it will be important for future studies to evaluate whether the early CD4+ T cell response predicts the quality and/or durability of the antibody response. over time. For example, follicular helper T cells (TFH) are thought to play a critical role in providing help to B cells and forming humoral immune memory, and circulating populations of these cells have been identified that correlate with the development of neutralizing antiviral antibodies9.
A recent paper demonstrated that the frequencies of circulating SARS-CoV-2-specific CD40L + OX40 + TFH cells were stable over several months. It will be important to know whether the magnitude or quality of circulating antigen-specific TFH cells or other cell types at early time points predicts the quality and/or durability of subsequent antibodies.
The fact that the majority of CD4 + and CD8 + T cells were activated by non-S viral epitopes has implications for vaccination in populations that have experienced previous SARS-CoV-2 infections. To activate these non-S-reactive T cells that were established during infections, proteins in addition to S could be incorporated into vaccines; this could enhance neutralizing and non-neutralizing antibody responses by eliciting a more robust CD4 + T cell response and would elicit a greater CD8 + T cell response that had been activated during prior infection.
Eliciting more robust antibody and CD8 + T cell responses against non-S epitopes during vaccination could be important, as there are likely targets of non-S immunity that promote resolution of infections and that may mediate broad immunity against variants of the SARS-CoV-2.
A central question related to SARS-CoV-2 immunity is whether infection establishes a reservoir of memory cells against this pathogen that are capable of defending against subsequent infection.
This study is reassuring, as most people who were infected six months earlier, even if they experienced no symptoms or mild symptoms during infection, were able to mount a cellular immune response against this pathogen.
However, significant heterogeneity in the T cell response was observed and therefore future studies will need to define whether the early T cell response predicts the quality of antibodies elicited by infection and what host factors or Viral variables other than initial disease severity predict the magnitude, quality and/or durability of immunity to SARS-CoV-2. From there, we can develop an understanding of the immune correlates of protection against long-term clinical outcomes such as post-acute COVID syndrome and/or reinfection, even with SARS-CoV-2 variants (Fig. 1) .
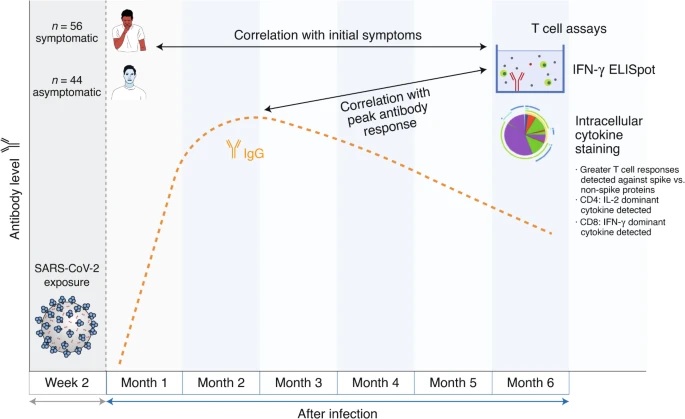
Zuo et al. measured SARS-CoV-2-specific T cell responses in 100 individuals six months after infection. SARS-CoV-2-specific T cell responses were measured by IFN-γ ELISpot and intracellular cytokine staining and correlated with both initial symptoms and maximal antibody response.