University of Leeds (UK)
A cheap and widely available prescription drug can improve symptoms of irritable bowel syndrome in patients seen in GP surgeries, according to new research presented today at UEG Week 2023 .
Summary Titrated Low-Dose Amitriptyline for Irritable Bowel Syndrome as Second-Line Treatment in Primary Care (ATLANTIS): Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial Background Most patients with irritable bowel syndrome (IBS) are treated in primary care. When first-line therapies for IBS are ineffective, UK National Institute for Health and Care Excellence guidelines suggest considering low-dose tricyclic antidepressants as second-line treatment, but their effectiveness in care is unknown. primary and are rarely prescribed in this setting. . Methods This randomized, double-blind, placebo-controlled trial ( Titrated Low-Dose Amitriptyline for Irritable Bowel Syndrome as Second-Line Treatment [ATLANTIS] ) was carried out in 55 general practices in England. Eligible participants were 18 years or older, had Rome IV IBS of any subtype, and persistent symptoms (IBS Severity Scoring System [IBS-SSS] score ≥75 points) despite changes in diet and medication therapies. first line, a normal complete blood count and C-reactive protein, negative celiac serology and no evidence of suicidal ideation. Participants were randomly assigned (1:1) to low-dose oral amitriptyline (10 mg once daily) or placebo for 6 months, with dose titration over 3 weeks (up to 30 mg once daily), according to symptoms and tolerability. Participants, their general practitioners, investigators, and analysis team were blinded to allocation throughout the trial. The primary outcome was the IBS-SSS score at 6 months. Efficacy analyzes were performed on an intention-to-treat basis; Safety analyzes were performed on all participants who took at least one dose of the trial medication. This trial is registered with the ISRCTN Registry (ISRCTN48075063) and is closed to new participants. Results Between October 18, 2019 and April 11, 2022, 463 participants (mean age 48.5 years [SD 16.1], 315 [68%] women and 148 [32%] men) were randomized to receive low doses. amitriptyline (232) or placebo (231). Intention-to-treat analysis of the primary outcome showed a significant difference in favor of low-dose amitriptyline in the IBS-SSS score between groups at 6 months (–27.0, 95% CI: –46.9 to –7.10; p=0·0079). 46 (20%) participants discontinued low-dose amitriptyline (30 [13%] due to adverse events) and 59 (26%) discontinued placebo (20 [9%] due to adverse events) before 6 months. There were five serious adverse reactions (two in the amitriptyline group and three in the placebo group) and five serious adverse events unrelated to trial medication. Interpretation To our knowledge, this is the largest trial ever conducted on a tricyclic antidepressant in IBS. Low-dose amitriptyline was superior to placebo as a second-line treatment for IBS in primary care across multiple outcomes, and was safe and well tolerated. General practitioners should offer low-dose amitriptyline to IBS patients whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-directed dose titration, such as the self-titration document developed for this trial. Money National Institute for Health and Care Research Health Technology Assessment Program (grant reference 16/162/01). |
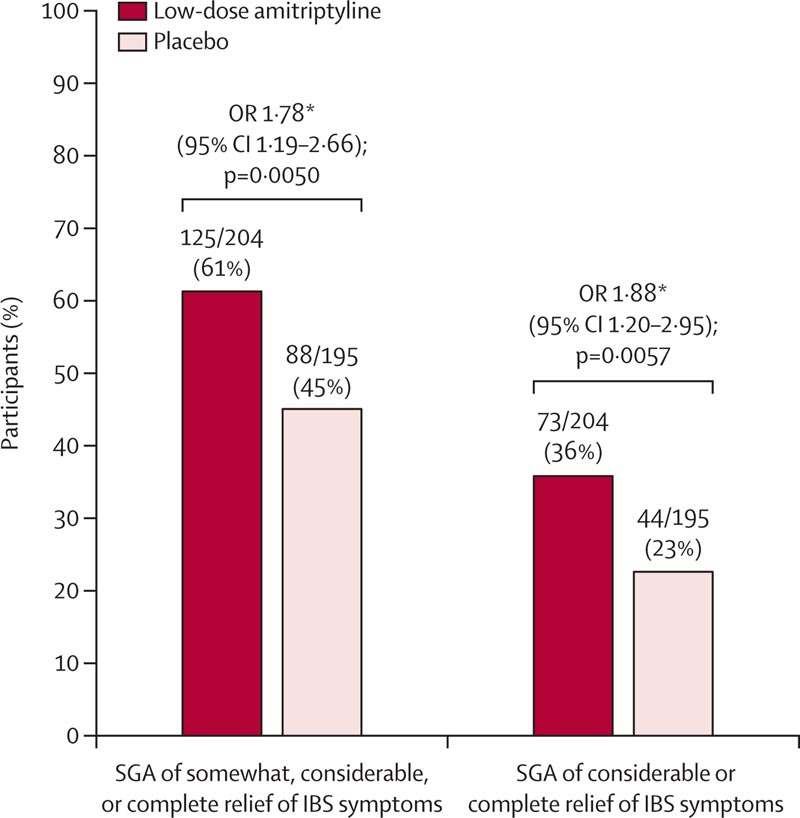
Figure: SGA key secondary outcome of IBS symptom relief at 6 months SGA=subjective global assessment. IBS = irritable bowel syndrome. OR=odds ratio. HADS=Hospital Anxiety and Depression Scale. *All ORs were estimated using logistic regression adjusted for recruiting center, IBS subtype, and HADS depression score. Missing data were imputed using multiple imputation.
Comments
According to results from the ATLANTIS trial, amitriptyline , which is commonly used in low doses for a variety of health problems, has also been found to improve symptoms of irritable bowel syndrome (IBS).
Led by researchers from the Universities of Leeds, Southampton and Bristol and funded by the National Institute for Health and Care Research (NIHR), the study was carried out in primary care. GPs prescribed the medication and patients administered their own dosage based on the severity of their symptoms, using a titration document designed for the trial. Most people with IBS are seen and cared for in primary care by their GP, meaning that the results of this trial are likely to be applicable to many people with this condition.
The results, published in The Lancet , showed that patients taking amitriptyline were almost twice as likely to report overall improvement in symptoms as those taking a placebo.
The trial team now recommends that GPs support their IBS patients to use amitriptyline to manage their symptoms and have made the dose adjustment document available to doctors and patients.
Co-chief investigator Alexander Ford, Professor of Gastroenterology at the University of Leeds School of Medicine, said: “Amitriptyline is an effective treatment for IBS and is safe and well tolerated. “This rigorously conducted new research indicates that general practitioners should help primary care patients try low-dose amitriptyline if their IBS symptoms have not improved with recommended first-line treatments.”
IBS, which affects about 1 in 20 people worldwide, causes abdominal pain and changes in bowel movements.
This long-term condition, which has no known cure, fluctuates in severity over time. It can have a substantial impact on quality of life and the ability to work and socialize. Most treatments have only a modest effect and people often have ongoing bothersome symptoms.
Amitriptyline belongs to a group of medications called tricyclics . They were originally used in high doses to treat depression, but are rarely used for this condition today because newer treatments have been developed.
Previous small trials of low-dose tricyclic antidepressants for IBS suggested a possible benefit in patients seen in hospital clinics, who often have more difficult-to-treat symptoms, but this new study is the first randomized controlled trial of low-dose amitriptyline versus a placebo tablet. for IBS in primary care. It is also the largest trial of amitriptyline for IBS conducted worldwide.
GPs already prescribe low-dose amitriptyline to treat chronic neuritic and back pain, and to help prevent migraine attacks. NICE guidelines currently state that GPs could consider using a low-dose tricyclic, such as amitriptyline, for IBS but, until now, evidence of a benefit has been uncertain.
Based on the trial results, which showed a clear benefit from amitriptyline, GPs may offer low doses of amitriptyline to people with IBS as part of shared decision making if symptoms do not improve with first-line treatments.
Co-principal investigator Hazel Everitt, Professor of Primary Care Research at the University of Southampton’s Primary Care Research Centre, said: "Before ATLANTIS, GPs did not typically prescribe amitriptyline for IBS because the research evidence was uncertain, but our new research provides good evidence of benefit.
"GPs already prescribe low-dose amitriptyline for other conditions, such as chronic pain and poor sleep, and when we interviewed GPs as part of this research, they were willing to prescribe it for IBS if evidence of The research backed it up. “Participants were also interested in having another option to try to relieve their IBS symptoms and most were happy to self-adjust their dose depending on symptoms and side effects.”
The ATLANTIS trial was funded by the NIHR Health Technology Assessment programme. Some 463 people with IBS from three regions of the UK took part: West Yorkshire, Wessex and the west of England. They were recruited from 55 general practices.
The participants were randomly divided into two groups: those who received amitriptyline and those who received a placebo. Participants controlled how many tablets of the trial medication they took and received support through the patient dosage adjustment document that was developed with patient representatives especially for this trial. This allowed participants to increase or decrease the number of tablets depending on their IBS symptoms and any side effects experienced.
Participants taking amitriptyline reported greater improvement in their symptom scores after six months compared to those taking a placebo. Those taking amitriptyline were almost twice as likely as those taking a placebo to report overall improvement in IBS symptoms, with amitriptyline performing better on a wide range of IBS symptom measures.
The researchers monitored the participants’ anxiety or depression scores and found that they were not altered , suggesting that the beneficial effects of the drug occurred through the gut , not due to any antidepressant-like effects.
No safety concerns were identified, and side effects in people taking amitriptyline were mostly mild, such as dry mouth in the morning.
Research in context
Evidence before this study
Most patients with irritable bowel syndrome (IBS) are treated in primary care. When first-line treatments such as changes in diet, fibre, laxatives or antispasmodic or anti-diarrheal drugs do not improve symptoms, the National Institute for Health and Care Excellence (NICE) guidance for the treatment of IBS in care Primary care in the UK suggests that clinicians should consider low-dose tricyclic antidepressants as second-line treatment.
We searched PubMed using the terms “irritable bowel syndrome,” “treatment,” and “tricyclic antidepressant” to identify articles published between January 1, 1980, and May 23, 2023. We did not limit the search based on drug dosage. tricyclic antidepressant studied or use language restrictions. We identified 168 articles that reported on this topic. Although several systematic reviews and meta-analyses report that tricyclic antidepressants are effective for IBS, all but one of the randomized controlled trials contributing data to these meta-analyses are small and underpowered, and none were conducted entirely in primary care. This calls into question the generalizability of their findings to patients in this context. Furthermore, the NICE guideline highlights the need to conduct a trial of low-dose tricyclic antidepressants in IBS in primary care.
We aimed to evaluate whether low-dose amitriptyline was effective as a second-line treatment for IBS in primary care in a pragmatic, randomized, double-blind, placebo-controlled trial.
Added value of this study
To our knowledge, this is the largest trial ever conducted of a tricyclic antidepressant in IBS, and the first based entirely in primary care. Over 6 months of treatment, low-dose amitriptyline , titrated from 10 mg to a maximum of 30 mg once daily, was superior to placebo for both primary and key secondary outcomes in 463 participants.
Amitriptyline was also superior to placebo on many other symptom-based outcomes for IBS, but had no impact on reporting of somatoform symptoms, anxiety, depression, or work and social adjustment scores at 6 months. Significantly more participants found it acceptable to take low doses of amitriptyline than placebo and almost three-quarters adhered to the drug during the trial, with adherence generally higher in the amitriptyline group.
Adverse events were more frequent with low doses of amitriptyline and in accordance with the known anticholinergic effects of the drug, but most were considered mild. Withdrawals due to adverse events were slightly more frequent with low-dose amitriptyline.
Implications of all available evidence
The results of this trial of titrated low-dose amitriptyline as a second-line treatment for IBS in primary care strongly support its use in this setting. General practitioners should offer low-dose amitriptyline to IBS patients whose symptoms do not improve with first-line therapies, with appropriate support to guide patient-directed dose titration, such as the self-titration document developed for this trial. Trials of amitriptyline as a first-line treatment for IBS in primary care would be informative.
Final message In conclusion, this trial of low-dose amitriptyline, 10 mg to 30 mg once daily, as second-line treatment in 463 participants with IBS in primary care has addressed an important unanswered question. Amitriptyline was more effective than placebo on a variety of IBS symptom measures, and was safe and well tolerated when titrated to symptom response and side effects. When the rationale for using a tricyclic antidepressant for IBS is clearly explained, as in the information materials provided to participants in this trial, with appropriate support, many people with IBS find it acceptable and beneficial. General practitioners should offer low-dose amitriptyline to IBS patients in whom first-line therapies are ineffective, with appropriate support to guide patient-directed dose titration, such as the self-titration document we developed. Management guidelines should be updated to reflect these findings. |















