Summary Metabolic comorbidities are common in patients with cardiorenal disease; they can cause atherosclerotic cardiovascular disease (ASCVD), accelerate progression, and negatively affect prognosis. Common comorbidities are type 2 diabetes mellitus (T2DM), obesity/overweight, chronic kidney disease (CKD), and chronic liver disease . The cardiovascular system, kidneys, and liver are linked to many of the same risk factors (e.g., dyslipidemia, hypertension, smoking, diabetes, and central/trunk obesity), and shared metabolic and functional abnormalities cause harm in all these organs through the superposition of pathophysiological factors. The COVID-19 pandemic has further complicated the management of cardiometabolic diseases. Obesity, T2DM, CKD and liver disease are associated with an increased risk of poor outcomes from COVID-19 infection and, conversely, COVID-19 may lead to worsening of pre-existing atherosclerotic cardiovascular disease (ASCVD). The high rates of these comorbidities highlight the need to improve the recognition and treatment of ASCVD in patients with obesity, insulin resistance or T2DM, chronic liver diseases and CKD and, equally, to improve the recognition and treatment of these diseases in patients with ASCVD. . Strategies to prevent and control cardiometabolic diseases include lifestyle modification, pharmacotherapy, and surgery. More programs are needed at the societal level to encourage a healthy diet and physical activity. Many pharmacotherapies offer mechanism-based approaches that can target multiple pathophysiological pathways between diseases. These include sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, selective mineralocorticoid receptor antagonists, and combined glucose-dependent insulinotropic peptide/glucagon-like peptide-1 receptor agonist. Nonsurgical and surgical weight loss strategies may improve cardiometabolic disorders in people living with obesity. New biomarkers under investigation may aid in the early identification of individuals at risk and reveal new treatment targets. |
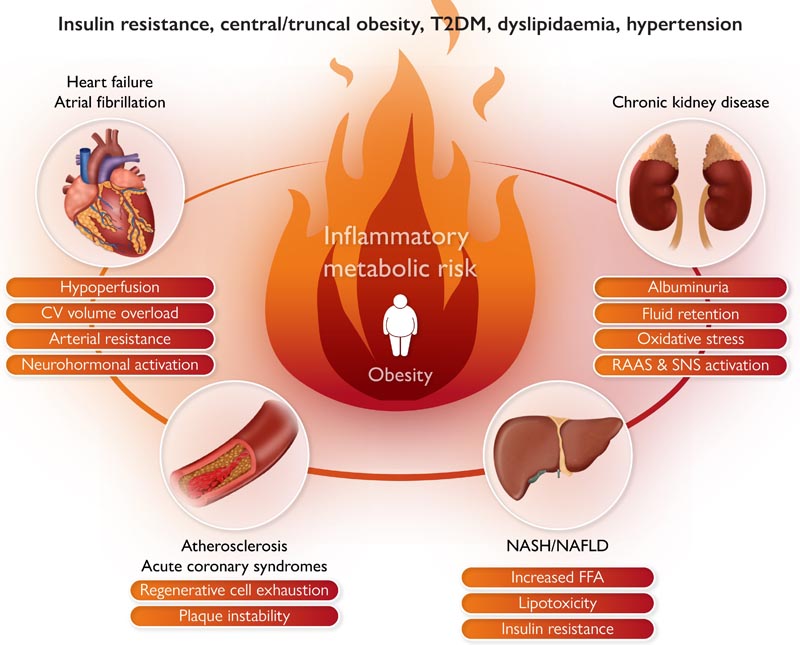
Graphical abstract: Obesity, particularly ectopic fat accumulation, has been linked to chronic inflammation and insulin resistance, which are linked to multiple cardiovascular risk pathways. CV, cardiovascular; FFA, free fatty acid; NAFLD, nonalcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system; DM2, type 2 diabetes mellitus .
Metabolic diseases are the cause of many cardiorenal diseases and can drive the progression of atherosclerotic cardiovascular disease (ASCVD) and negatively affect prognosis. Despite this, the comorbid diagnoses of type 2 diabetes mellitus (T2DM), chronic kidney disease (CKD), and chronic liver disease are often overlooked. These disorders have many risk factors in common , such as insulin resistance, hypertension, obesity, and dyslipidemia. There is a need to improve the recognition of cardiovascular disease (CVD) and kidney disease in patients with metabolic disorders and the recognition of metabolic disorders in patients with cardiorenal diseases.
Cardiometabolic conditions play an important role in patients with COVID-19 infections. An increased risk of serious infection and mortality has been associated with CVD, obesity, T2DM, CKD, and liver disease. Additionally, COVID-19 infection can cause worsening of pre-existing CVD. The virus damages many organs, including the heart and blood vessels, and can promote blood clots, myocardial infarction (MI), and heart inflammation.
Studies have reported substantial overlap in the pathophysiological mechanisms of these diseases and syndromes, suggesting that a mechanism-based intervention approach may be useful across multiple disorders. Interventions used for the treatment of patients with T2DM, including sodium-glucose cotransporter 2 (SGLT-2) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1 RA), selective mineralocorticoid receptor antagonists (SMRAs) ), and bariatric surgery, have demonstrated beneficial effects in the prevention and treatment of CKD and CVD beyond their reductions in body weight, glycated hemoglobin (HbA1c), and blood pressure.
This article was developed from presentations and discussions during a hybrid cardiovascular roundtable workshop (in-person and virtual participants) organized by the European Society of Cardiology (ESC). It aims to explore challenges and barriers in the recognition and treatment of patients with multiple cardiometabolic comorbidities and examine potential mechanism-based approaches to intervention across multiple disorders. These patients require a comprehensive approach using a combination of pharmacological and non-pharmacological approaches for prevention, diagnosis and treatment.
Prevalence and impact of cardiovascular diseases and metabolic conditions
Among the most common overlapping cardiometabolic comorbidities are CVD, T2DM, obesity/overweight, kidney disease, and liver disease. Globally in 2019, there were 438 million prevalent cases and 1.5 million deaths from type 2 diabetes (T2DM). Globally, it is estimated that 50% of deaths among patients with T2DM are due to CVD. Diabetes is associated with an increased risk of all-cause mortality, CVD, stroke, CKD, chronic liver disease, and cancer.
Adults with T2DM have a two- to four-fold increased risk of CVD compared to those without diabetes.
Obesity is a common risk factor for hypertension, CVD, CKD, and T2DM . The prevalence of overweight (30%–40%) and obesity (26%–27%) is high and continues to increase in both developed and developing countries. Obesity promotes inflammation, hypertension, insulin resistance, and impaired cardiac and vascular function and has been associated with an increased risk of cardiometabolic diseases and mortality in the general population. Globally, high body mass index (BMI) accounted for 5.0 million deaths and 160 million disability-adjusted life years (DALYs), of which more than half were due to CVD (2019).
However, overweight/obesity per se does not adequately reflect the different obesity phenotypes and their corresponding risk profiles. While the general term “obesity” has been used throughout this manuscript (and in most of the studies cited), the distribution of adipose tissue is also important. There is increasing evidence that genetically predicted adipose tissue distribution influences ASCVD risk and that gluteofemoral adipose tissue may even be protective . Weight gain resulting in intra-abdominal fat , including visceral depots, may be predominantly related to adipocyte hypertrophy , while increased lower body fat, including gluteofemoral tissue, may be related to hyperplasia . It has been suggested that waist circumference, waist-to-height ratio, or waist-to-hip ratios may be better markers of adiposity than BMI.
The waist-height ratio has shown a good predictive value for detecting insulin resistance/hyperinsulinemia .
In studies that have classified obesity according to metabolic health , people with metabolically healthy obesity have a lower cardiovascular risk compared to those with metabolically unhealthy overweight or obesity . However, the majority of metabolically healthy obese individuals converted to unhealthy phenotypes during long-term follow-up (median 24 years).
Liver disease
Globally, in 2019, there were 1.24 billion cases and 134,000 deaths, and 3.62 million cases of atherosclerotic cardiovascular disease (ASCVD) due to cirrhosis and other chronic liver diseases due to non-alcoholic fatty liver disease (NAFLD ). NAFLD) and non-alcoholic steatohepatitis (NASH). Nonalcoholic fatty liver disease (NAFLD) is associated with a high prevalence of comorbid metabolic conditions including obesity (51%), T2DM (23%), hyperlipidemia (69%), hypertension (39%), and metabolic syndrome (43 %). The risks of obesity, hypertension and DM2 are greater as the stage of liver fibrosis increases. The prevalence of NAFLD in patients with T2DM based on transient elastography has been estimated to be around 70% to 85%, with moderate liver fibrosis in 6% to 27% of patients and advanced liver fibrosis in 9% to 13%.
The underlying associations between NAFLD and T2DM are bidirectional, with NAFLD sometimes preceding or exacerbating the development of T2DM (indeed, liver fat appears to be causally related to diabetes) and promoting adverse outcomes associated with diabetes. While CVD is the most common cause of death in these patients, accounting for 40% of deaths, NAFLD itself does not appear to be an independent risk factor for myocardial infarction and stroke; however, with the high prevalence of common risk factors, a thorough risk assessment is warranted.
Renal disease
At least half of T2DM patients worldwide also have Chronic Kidney Disease (CKD). The 10-year cumulative all-cause mortality rate in patients with T2DM and CKD is 31%, compared to 12% with T2DM alone and 8% without either condition. While diabetes alone has been shown to reduce life expectancy by an average of about 6 years in high-income countries, the presence of comorbid CKD can shorten life expectancy by 16 years.
Kidney failure is also associated with an increased risk of hospitalization for heart failure (HF). Obesity has been associated with the development and progression of CKD. Compared with those of normal weight, the relative risk of kidney disease in obese patients is almost double and that of those who are overweight is 1.40 times higher. Obesity is upstream of type 2 diabetes and hypertension that may drive CKD progression, but there is also good genetic evidence that obesity is causally related to CKD, albeit with varying relative weights.
Heart disease
Approximately half of patients with Heart Failure (HF) have CKD. CKD is associated with a 50% increased risk of death in HF with reduced ejection fraction (HFrEF) and HF with mildly reduced ejection fraction (HFmrEF) and a 30% increased risk of death with preserved ejection fraction (HFpEF). This suggests that CKD may have a somewhat different background in HFrEF and HFmrEF (e.g., partly related to perfusion and congestion) than in HFpEF (where CKD and HFpEF may arise more from common underlying comorbidities , such as DM2 and obesity).
Shared mechanisms of comorbid conditions
As described above, there are different phenotypes of obesity , and the accumulation of visceral adipose tissue is related to elevated CV risk. Ectopic fat accumulation in the abdominal cavity (visceral fat), as well as in the pericardium, liver, and pancreas, has been linked to chronic inflammation and insulin resistance , which are linked to multiple cardiovascular risk pathways ( Graphic summary). Excess liver fat production may be a common early pathway in the development of NAFLD and ASCVD in high-risk patients. Body composition , including ectopic fat accumulation (visceral adipose tissue), combined with age-related muscle loss, can lead to hyperinsulinemia, atherogenic dyslipidemia, ongoing inflammation, endothelial dysfunction, and impaired fibrinolysis .
Insulin resistance can lead to impaired beta cell function and plays an important role in the development of T2DM. Glucose from carbohydrates is stored in the muscle and liver as glycogen, and insulin resistance in these organs results in hyperglycemia. Studies have implicated ectopic lipid accumulation in the pathogenesis of insulin resistance that can lead to nonalcoholic fatty liver disease (NAFLD), atherogenic dyslipidemia, and ultimately CVD. Insulin resistance plays a role in obesity-related heart failure (HF) through the impact of altered metabolic insulin signaling, reduced bioavailable nitric oxide, and increased oxidative stress and inflammation, leading to myocardial tissue remodeling and interstitial fibrosis.
Using LDL cholesterol (LDL-C) alone may underestimate the risk of ASCVD in people with visceral adiposity and other features of the metabolic syndrome. Non-HDL cholesterol (HDL-C, total cholesterol minus HDL-C), which includes not only LDL-C but also very LDL and atherogenic remnant lipoproteins, may be a better indicator of ASCVD risk than LDL-C, particularly in patients with an ectopic fat phenotype. The ratio of triglycerides to HDL-C is associated with insulin resistance and atherogenic dyslipidemia and may also be useful. An elevated waist circumference is predictive of ectopic and hepatic fat, which are independent risk factors for ASCVD and mortality.
The combination of elevated triglycerides and waist circumference improves risk prediction.
C-reactive protein is a downstream marker of the interleukin (IL)-1beta-IL-6 pathway, which plays a role in ASCVD and may be a useful adjunctive measure in patients with metabolic syndrome. Other common abnormalities associated with ectopic fat include intermediate liver fat [alanine aminotransferase (ALT)/gamma-glutamyl transferase] levels and HbA1c levels.
Obesity may also affect CVD risk through obstructive sleep apnea , which leads to hypoxia and is associated with cardiac arrhythmias, insulin resistance, and hypertension. Additionally, there are direct effects of weight gain on mobility and physical activity, which can exacerbate weight gain and cardiovascular risk. Obesity leads to increased blood volume and cardiac output, which can lead to structural and functional changes in the heart.
Obesity also contributes to cardiorenal syndromes through insulin resistance, hypertension, and dyslipidemia. Reduced kidney function, albuminuria, and kidney disease progression have also been associated with obesity, independent of insulin resistance. The mechanism for this may be related to glomerular hyperfiltration , which has been linked to adiposity and hypertension. Hyperfiltration depends on visceral fat production of inflammatory adipokines and factors that stimulate aldosterone production in the zona glomerulosa of the adrenal cortex. The effect on kidney disease progression is also related to the activation of inflammation and oxidative stress pathways.
Type 2 diabetes (T2DM) and high blood pressure are involved in the progression of chronic kidney disease (CKD) leading to accelerated atherosclerosis, progressive left ventricular hypertrophy, and development of heart failure (HF). Chronic cardiorenal syndromes are related in part to fibrosis, left ventricular hypertrophy, vascular stiffness, chronic sodium and volume overload, other common neurohumoral and inflammatory mechanisms, and oxidative stress injury. Obesity causes upregulation of the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system, which also contribute to the risk of high blood pressure and stroke.
Impact of COVID-19
Cardiometabolic diseases affect COVID-19 infection
Obesity, T2DM, CKD, and liver disease were found to be significantly associated with an increased risk of severe COVID-19, including mortality. Many factors associated with an increased risk of severe COVID-19 infection were cardiovascular risk factors, such as hypertension, obesity, and T2DM. In one study, T2DM was associated with a 6.3-fold increased risk of death, which was substantially greater than the increased risk associated with cardiovascular conditions (1.2 to 1.4).
Potential mechanisms underlying the interaction between metabolic conditions (i.e., obesity and T2DM) and COVID-19 infection may be related to cardiovascular, respiratory, metabolic, and thrombotic deficiencies intrinsic to obesity and excess fat. ectopic. These perturbations in relation to ectopic fat likely reduced the patient’s ability to cope with COVID-19 infection and the secondary immune reaction. Patients living with obesity may have amplified or dysregulated immune responses due to increased viral exposure, which may be enhanced by excess adipose tissue. Parallels between COVID-19 pathology and T2DM suggest that pre-existing dysfunctional inflammatory responses, glycemic deficiencies, and tissue injury along with excess upstream adiposity may predispose patients to worse outcomes from COVID infection. -19 (Table 1).
CVD has also been associated with worse outcomes among people with COVID-19 infection, including increased risk of mortality and secondary cardiovascular or renal events. Patients with HF who were hospitalized with COVID-19 infection had an approximately two-fold increased risk of mortality compared with those without a history of HF and a 10-fold increased risk compared with those hospitalized with acute HF without COVID-19. Similarly, in a large UK cohort study in patients hospitalized with COVID-19, pre-existing cardiometabolic comorbidities were associated with an increased risk of death [odds ratio (OR) 1.21; 95% confidence interval (CI): 1.16–1.27] and in-hospital complications (OR 2.54; 95% CI: 2.43–2.65), and the risks increase with increasing number of comorbidities.
COVID-19 affects cardiometabolic diseases
In severe COVID-19 infection, death is often due to lung disease; However, the virus damages many other organs, including the heart and blood vessels, and can promote the formation of blood clots, myocardial infarction, and heart inflammation. The relative risks of myocardial infarction and stroke within 14 days after diagnosis of COVID-19 infection were 13 and 6 times higher compared to before or 14 days after COVID-19 diagnosis. The underlying mechanisms likely include cytokine-mediated plaque destabilization , hypercoagulability , and increased myocardial oxygen demand , rather than a direct cardiotropic effect.
There was also evidence of an increase in myocarditis from COVID-19 infection, but this was very modest. For example, a systematic review suggested a five-fold increase in the risk of myocarditis in those who survived COVID-19 compared to people who had not had COVID-19, with an absolute increase of about 1 in 10,000. Elevated risks of myocarditis were also associated with some vaccines, particularly in younger men, but such risks were much lower than with COVID-19 infection.
In addition to the direct effects of viral infection, the COVID-19 pandemic also had a serious impact on cardiometabolic diseases as a result of disrupted access to healthcare. The large increases in mortality during the pandemic were not explained by COVID-19 deaths alone.
During the pandemic, there was a significant decrease in hospitalizations for CVD and, interestingly, also a reduction in ventricular arrhythmias requiring device intervention, possibly due to reductions in "real-life stressors" and physical activity during the pandemic. One study found a decrease in elective procedures during the pandemic, but no change in the number of urgent angiograms or percutaneous coronary interventions, suggesting that patients were presenting to the hospital later and with more severe ischemia. Patients have likely been avoiding hospitals for fear of contracting the virus.
The pandemic also had a substantial impact on the management of type 2 diabetes (T2DM), including missed or delayed diagnoses, inadequate control of HbA1c levels, and postponement of follow-up care. In a national study of cardiovascular drug prescribing during the pandemic, substantial reductions in prescriptions of antihypertensive and lipid-lowering drugs (much more than glucose-lowering drugs) were observed with an increased predicted risk of future myocardial infarction and stroke . The authors suggested the need for methods to identify and treat people with CVD risk factors to prevent excess future CVD events.
Management strategies in patients with COVID-19
Patients with pre-existing or post-COVID-19 CVD should be treated according to current guidelines, such as those for acute coronary syndromes, heart failure (HF), or arterial hypertension. Concerns related to the use of RAAS inhibitors and the risk of acute COVID-19 have proven to be unfounded , and these agents should be continued in patients with stable CVD. In patients with T2DM, a Swedish registry study found that patients who had been prescribed SGLT-2 inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors, but not GLP-1 RA, had a slightly increased risk (≤11%) of severe COVID-19 infections. However, the Dapagliflozin in Respiratory Failure in Patients with COVID-19 trial did not find a significant impact of an SGLT-2 inhibitor on the risk of organ dysfunction or death, but the drug was well tolerated and there were no safety concerns.
In patients with heart failure, withdrawal of guideline-directed medical therapy has been associated with increased mortality, so therapy should be continued and optimized. However, optimal strategies for managing post-COVID sequelae and increased CVD risk have not yet been defined. That said, it is clear that an adverse legacy of the pandemic on cardiovascular risks and their management has occurred and that this may have attenuated some of the gains in cardiovascular deaths seen over the past 3 to 4 decades.
Mechanism-based approaches to identify pathology in all diseases
Interventions at the societal and individual level to address common risk factors
One of the key social strategies to combat obesity and cardiometabolic diseases is education and programs to encourage a healthy diet and physical activity. An unhealthy diet, such as exposure to fast food establishments, has been associated with an increased risk of obesity. Strategies should promote increased consumption of fresh fruits and vegetables and decreased consumption of sugary foods and beverages (Table).
The European Union (EU) School Fruit and Vegetable Program has been shown to be effective in significantly increasing children’s fruit and vegetable intake, as well as their nutritional attitudes and behaviours. Similarly, food subsidies can increase sales of fruits and vegetables. There is also evidence of the beneficial effects of sugar taxes, with reductions in the purchase of sugary drinks leading some soft drink manufacturers to reformulate their products.
UK Chief Medical Officer’s Summary of Strategies to Tackle Childhood Obesity, 2019 1) Regulate the balance of foods sold to favor healthy options. 2) Eliminate marketing, signs and incentives that encourage children to consume unhealthy foods and drinks. 3) Introduce innovative policies that balance children’s health and the needs of the private sector. 4) Design and invest in the development of an environment that allows children to be active and healthy. 5) Encourage exercise and healthy weight during pregnancy and encourage breastfeeding. 6) Give a central role to the school system, supported by school inspection policies. 7) Ensure a healthcare workforce that can provide what children and families need to prevent and manage obesity, including education about weight and weight stigma. 8) Optimize the use of available data and technology to guide practice. 9) Protect the health and rights of children when making business agreements. 10) Continue to develop a strong evidence base to inform practice and policy. |
In addition to societal-level interventions, individual-level interventions have shown that even mild physical activity programs can reduce adiposity and improve blood pressure and lipid profiles. Meta-analysis of six prospective observational studies found that people who spent more time in light activities had a lower risk of all-cause mortality [hazard ratio (HR) 0.71; 95% CI, 0.62-0.83].
Similarly, a meta-analysis of randomized controlled trials (RCTs) found that resistance training reduced blood pressure, fasting insulin, and insulin resistance, particularly in people with high risk or cardiometabolic disease. . Physical inactivity is one of the main contributors to cardiovascular disease. A systematic review of 36 studies reported that moving from being inactive to achieving the recommended 150 min/week of moderate-intensity aerobic activity was associated with lower risks of CVD mortality (23%), CVD incidence (17%), and T2DM ( 26%).
A systematic review of 32 prospective studies reported that plant protein-based diets were associated with a lower risk of cardiovascular and all-cause mortality. Importantly, dietary patterns differ with respect to reducing ectopic fat depots, with a carbohydrate-restricted diet being more effective than a low-fat dietary pattern in mobilizing specific ectopic fat depots. Therefore, dietary patterns have weight-independent effects on negative body composition associated with CVD.
Based on numerous lifestyle studies, the US Preventive Services Task Force recommends behavioral counseling interventions to improve diet and increase physical activity for people with cardiometabolic risk factors to prevent cardiovascular events at risk. long term.
Lifestyle modification programs should also encourage physical activity and provide education about the cardiometabolic risks and benefits of a healthy lifestyle. Such programs require changes in legislation and policies. The Organization for Economic Co-operation and Development (OECD) (www.oecd.org), an international organization with 38 member countries, works to promote better policies for better lives, including by addressing risk factors for cardiometabolic and other diseases. not transmissible.
In an analysis of 52 countries, 51 had national policies to address adult obesity, and the vast majority had national guidelines on diet and physical activity. Other policies include mandatory nutrition standards in schools, restrictions on television advertising, taxes on health-related foods (mainly on sugary drinks), and mandatory front-of-package labeling. Evidence from modeling studies suggests that investing in prevention strategies will prevent hundreds of thousands of CVD cases over the next 30 years, generate millions of dollars in healthcare savings per year, and increase workforce productivity.
Patient experience
Participating patients agreed that there was an urgent need for education, prevention and screening . More than any other disease, chronic conditions such as T2DM and cardiovascular disease require close doctor-patient collaboration to effectively manage risk factors and disease. It is essential that healthcare professionals know the intimate details of the lived experiences of patients with CVD and comorbidities.
Key messages from participating patients to physicians and the public highlighted the need for more education to ensure that physicians perform rigorous screening for multiple conditions. Additionally, the public must be educated about the devastating consequences associated with T2DM and the need for screening. Patients emphasized the need for cultural change, including health education at an early age.
A more holistic approach to patient management is needed, with continued interaction between cardiologists and other healthcare professionals. For example, cardiologists should refer patients for screening for related conditions after experiencing a cardiovascular event. Clinics dedicated to preventive cardiology have been suggested as a way to facilitate this; however, many patients may not have access to such resources.
Professional cardiology societies, including the ESC with its European Association for Preventive Cardiology and the American College of Cardiology, have proposed establishing a subspecialty dedicated to preventive cardiology . These specialists would be trained to address all aspects of cardiovascular health, including physical activity, nutrition and weight management, smoking cessation, psychosocial and behavioral factors, as well as environmental, genetic and risk factors. biologicals, and cardioprotective medications.
Detection of comorbid conditions
The high rates of comorbidity highlight the need to improve the recognition and treatment of cardiovascular diseases in patients with obesity, T2DM, chronic liver diseases and CKD and, conversely, improve the recognition and treatment of metabolic and kidney diseases in patients who attend cardiology consultation. Guideline recommendations for screening patients with cardiometabolic diseases are shown in Table 3. A 2022 statement from the American Diabetes Association also recommends biomarker screening using B-type natriuretic peptide (BNP), N-terminal pro-BNP. or high-sensitivity cardiac troponin , to detect HF in patients with DM2.
AF, atrial fibrillation; CAD, coronary artery disease; CKD, chronic kidney disease; CVD, cardiovascular disease; ECG, electrocardiogram; eGFR: estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OGTT: oral glucose tolerance test; DM2, type 2 diabetes mellitus; UACR, urine albumin:creatinine ratio .
Pharmacotherapy to address multiple pathologies in cardiometabolic diseases
Several therapeutic options may offer mechanism-based approaches that can address multiple pathologies across diseases, most notably lifestyle modifications including diet and physical exercise (discussed above), pharmacotherapies including statins, inhibitors of SGLT-2, GLP-1 RA, SMRA and bariatric surgery.
Among the most important pharmacotherapies that have been shown to reduce cardiovascular risk are statins . The ESC guidelines for the prevention of CVD and for the treatment of dyslipidemia recommend treatment with statins for primary and secondary prevention . Statins have been shown to reduce major vascular events (MI, death from coronary artery disease, or any stroke or coronary revascularization) and total mortality.
Statins have been shown to reduce systemic inflammation and induce regression of epicardial adipose tissue independently of their lipid-lowering effects. In addition to its cardiovascular benefits, several meta-analyses have demonstrated beneficial effects on liver and kidney parameters.
In a meta-analysis of 4 RCTs (n = 169) in patients with NAFLD and NASH, statins were shown to have lower levels of serum aspartate transaminase, alanine aminotransferase, triglycerides, and cholesterol compared with control groups. Similarly, a meta-analysis of 33 RCTs (n= 37,391) in patients with chronic kidney disease (CKD) concluded that the use of statins can delay the progression of CKD by significantly reducing urinary albumin and protein excretions and decreasing blood loss. of glomerular filtration rate (GFR) compared to control groups.
Several therapies for the treatment of T2DM have been shown to target the hemodynamic and metabolic mechanism involved in the development of CKD. Data show that treating multiple risk factors (glucose, blood pressure, and lipids) toward targets and treatment with RAAS inhibitors can decrease the risk of end-stage renal disease (ESRD) and death, and those who achieve three or Most targets have almost 60% risk reduction.
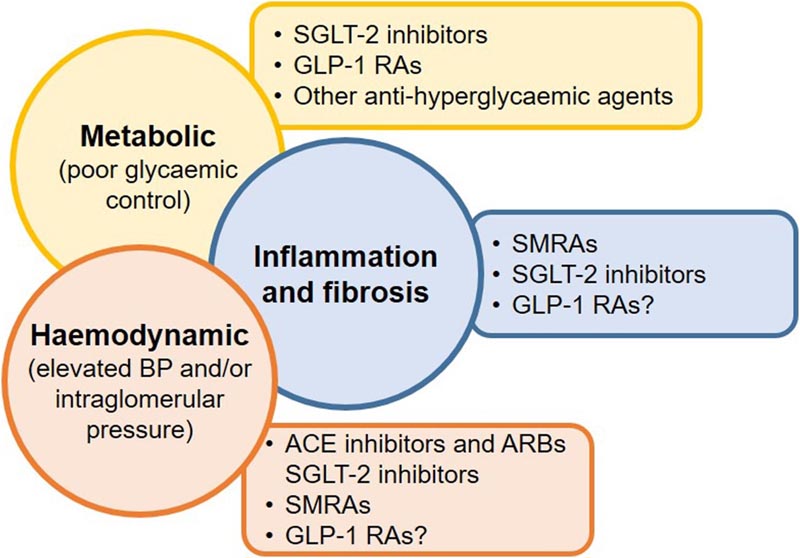
Figure Targeting multiple drivers of chronic kidney disease in type 2 diabetes mellitus with different therapies may improve outcomes. References are as follows: hemodynamics: ACE and ARB inhibitors, SGLT-2 inhibitors, SMRA. Metabolic: SGLT-2 inhibitors, GLP-1RA, other antihyperglycemic agents. Inflammation and fibrosis: SMRA. GLP-1 RA, glucagon-like peptide 1 receptor agonist; SGLT-2, sodium glucose cotransporter-2; SMRA, selective mineralocorticoid receptor antagonist .
Meta-analyses of clinical trials have demonstrated the efficacy of SGLT-2 inhibitors, 23 GLP-1 RAs, and selective mineralocorticoid receptor antagonists (SMRAs) on multiple cardiovascular and renal outcomes in patients with T2DM with or without CKD, beyond their reductions in body weight and HbA1c. The glucose-dependent insulinotropic peptide (GIP)/GLP-1 RA, tirzepatide, may also be useful, but more data are needed.
However, some hypoglycemic drugs promote adipogenesis and epicardial fat accumulation (e.g., insulin and insulin secretagogues that act on pancreatic beta cells such as sulfonylureas), while others, such as DPP-4 inhibitors, and GLP-1 RAs reduce ectopic fat accumulation but do not reduce inflammation . In contrast, statins, metformin, SGLT-2 inhibitors, and selective mineralocorticoid receptor antagonists (SMRAs) can reduce ectopic fat accumulation and inflammation, as well as its adipokine secretion.
SGLT-2 is expressed in epicardial adipose tissue and in vitro studies have shown that SGLT-2 inhibitors can increase glucose uptake and reduce the secretion of proinflammatory chemokines from epicardial adipose tissue and can improve endothelial cell healing. coronaries. Furthermore, a small study in nine nonobese patients with T2DM and visceral adiposity found that SGLT-2 inhibitors reduced epicardial fat volume.
Since GLP-1 receptor mRNA transcripts have been detected in human cardiomyocytes, part of the cardiovascular benefit of AR-GLP1 glucagon-like peptide 1 receptor agonists may be a direct effect of GLP-1 on the myocardium. GLP-1 infusion has been shown to improve left ventricular function in patients with acute MI after successful reperfusion and in patients with HF. In mouse models of acute MI, RA GLP-1 treatment demonstrated cardioprotective effects, and in HFpEF mice, treatment improved cardiac function, with reduced cardiac hypertrophy and myocardial fibrosis.
Surgical and non-surgical weight loss strategies targeting multiple pathologies in cardiometabolic diseases
Surgical and non-surgical strategies have been shown to reduce weight and improve cardiometabolic disorders in many obese people, particularly in terms of delaying the progression of prediabetes to T2DM and in the treatment of high blood pressure.
Matched cohort studies in adults with and without T2DM strongly suggest that bariatric surgery is associated with a lower risk of all-cause mortality. In meta-analyses of observational studies in obese individuals, surgery was associated with lower mortality rates by 45%–50% compared with matched individuals who did not undergo surgery. Prospective cohort studies suggest that bariatric surgery is more effective than lifestyle and intensive medical treatment alone. However, the long-term sustainability of benefits remains an issue. In a meta-analysis of 31 RCTs, although gastric bypass was associated with a 95% greater likelihood of complete or partial diabetes remission at 5 years compared with medical treatment, these rates progressively decreased over time, regardless of the intervention. .
Surgical and non-surgical weight loss can also reduce the progression of chronic kidney disease (CKD). A meta-analysis of 13 studies in patients with CKD found that non-surgical interventions (diet, exercise, pharmacotherapy) reduced BMI, proteinuria and systolic blood pressure (SBP) and in people with obesity surgical interventions reduced BMI, normalized glomerular filtration rate (GFR) and decrease in microalbuminuria and SBP. Similarly, a meta-analysis of observational studies in patients with T2DM found that bariatric surgery improved albuminuria. Patient registry data have shown a 70% decrease in the incidence of severe CKD during long-term follow-up (median 18 years) with bariatric surgery compared with usual obesity care. Surgical weight loss has also been associated with a 12.4% absolute risk reduction of major adverse liver outcomes.
Several meta-analyses have also found improvements in multiple cardiovascular risk factors in patients with obesity and T2DM undergoing bariatric surgery. Surgery was more effective than medical treatment in improving glycemic control, HDL-C, and triglyceride concentrations. Furthermore, large cohort studies reported significantly lower risks of cardiovascular events in patients with T2DM and severe obesity undergoing bariatric surgery compared to medical treatment.
However, RCTs demonstrating an impact on hard cardiovascular outcomes are lacking . One of the few trials with long-term follow-up (median 9.6 years) and primary cardiovascular outcomes was the Look AHEAD trial that compared intensive lifestyle intervention (diet and physical activity) with supportive diabetes care. The trial found that modest weight loss did not significantly reduce the incidence of cardiovascular events or mortality in overweight or obese patients with T2DM. Although weight loss was associated with improvements in HbA1c and other cardiovascular risk factors. Furthermore, post hoc subgroup analyzes reported a significantly lower risk of cardiovascular outcomes among those achieving >10% weight loss and those with inadequately controlled T2DM. Other analyzes of observational data have found that minimum weight loss thresholds for reducing the risk of cardiovascular events in patients with obesity and T2DM were ≥10% of body weight with surgical intervention and ≥20% with nonsurgical intervention.
Several pharmacological agents have demonstrated dramatic reductions in weight, including the SGLT-2 inhibitor, semaglutide, and the GIP/GLP-1 RA, tirzepatide. The effect of these agents on cardiovascular outcomes is currently being studied in Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (NCT03574597), the study of tirzepatide compared with dulaglutide on major cardiovascular events in participants with type 2 diabetes ( NCT04255433) and the tirzepatide study on reducing morbidity and mortality in adults with obesity (NCT05556512).
The guidelines on T2DM and CVD from the ESC and the European Association for the Study of Diabetes recommend weight control and physical activity to prevent T2DM and cardiovascular events (Table).
Guideline recommendations for lifestyle modifications to prevent CVD in patients with T2DM and prediabetes
Abbreviations : CV, cardiovascular; DM2, type 2 diabetes mellitus. |
Summary and call to action Steps are needed to address challenges and barriers to early identification, prevention, recognition, and treatment of cardiometabolic comorbidities. Strategies are required, including education, screening and diagnosis, and optimal use of available treatments. Strategies to address challenges and barriers in the prevention, recognition and treatment of cardiometabolic comorbidities Target lifestyle modifications
To help maximize patient acceptance of lifestyle interventions to address risk factors, provide education to healthcare professionals on how to convey risks and benefits to patients.
Target detection and diagnosis
Aim for cross-specialty care
Aim for optimal use of current therapies
Abbreviations : GIP, glucose-dependent insulinotropic peptide; GLP-1 RA, glucagon-like peptide 1 receptor agonist; PCPs, primary care physicians; SGLT-2, sodium glucose cotransporter-2; SMRA, selective mineralocorticoid receptor antagonist . |