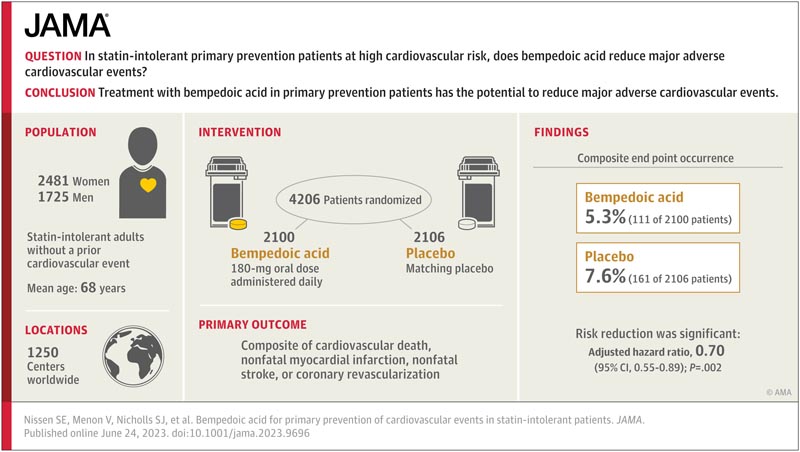
Key points In primary prevention patients intolerant to statins and at high cardiovascular risk, does bempedoic acid reduce major adverse cardiovascular events? Findings In this randomized trial of 13,970 patients, 4206 participants at high cardiovascular risk but without a prior cardiovascular event were enrolled. In this subgroup, treatment with bempedoic acid, 180 mg daily, was associated with a significant reduction in major cardiovascular events (hazard ratio, 0.70). Meaning These findings suggest that treatment with bempedoic acid in primary prevention patients has the potential to reduce major adverse cardiovascular events. |
Importance
The effects of bempedoic acid on cardiovascular outcomes in statin-intolerant patients without a prior cardiovascular event (primary prevention) have not been fully described.
Aim
To determine the effects of bempedoic acid on cardiovascular outcomes in primary prevention patients.
Design, environment and participants
This blinded randomized clinical trial enrolled 13,970 statin-intolerant patients (enrollment December 2016 to August 2019 at 1250 centers in 32 countries), including 4206 primary prevention patients.
Interventions
Participants were randomly assigned to oral bempedoic acid, 180 mg daily (n = 2100) or matching placebo (n = 2106).
Main outcome measures
The primary measure of efficacy was the time from randomization to the first occurrence of any component of a composite of cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, or coronary revascularization.
Results
The average age of the participants was 68 years , 59% were women and 66% had diabetes.
From a mean baseline value of 142.5 mg/dL, compared with placebo, bempedoic acid reduced low-density lipoprotein cholesterol levels by 30.2 mg/dL (21.3%) and of high-sensitivity C-reactive protein at 0.56 mg/L (21.5%), from a reference median of 2.4 mg/L.
Follow-up for a median of 39.9 months was associated with a significant risk reduction for the primary endpoint (111 events [5.3%] vs 161 events [7.6%]; adjusted hazard ratio [HR ], 0.70 [95% CI, 0.55-0.89]; P = 0.002) and key secondary endpoints, including the composite of cardiovascular death, myocardial infarction, or stroke (83 events [4, 0%] vs. 134 events [6.4%]; HR, 0.64 [95% CI, 0.48-0.84]; P < .001); MI (29 events [1.4%] vs 47 events [2.2%]; HR, 0.61 [95% CI, 0.39-0.98]); cardiovascular death (37 events [1.8%] vs 65 events [3.1%]; HR, 0.61 [95% CI, 0.41-0.92]); and all-cause mortality (75 events [3.6%] vs 109 events [5.2%]; HR, 0.73 [95% CI, 0.54-0.98]).
There was no significant effect on stroke or coronary revascularization.
Adverse effects of bempedoic acid included a higher incidence of gout (2.6% vs. 2.0%), cholelithiasis (2.5% vs. 1.1%), and increases in serum creatinine, uric acid, and Hepatic enzymes.
Conclusions
In a subgroup of high-risk primary prevention patients, bempedoic acid treatment was associated with a reduction in major cardiovascular events.
Reference : Bempedoic Acid for Primary Prevention of Cardiovascular Events in Statin-Intolerant Patients. Steven E. Nissen, Venu Menon, Stephen J. Nicholls, et al. JAMA. Published online June 24, 2023. doi:10.1001/jama.2023.9696