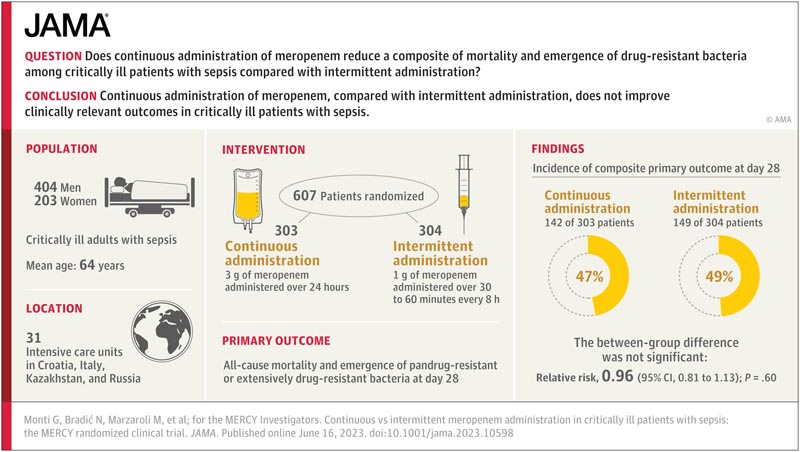
Key points Does continuous administration of meropenem reduce a combination of mortality and emergence of drug-resistant bacteria among critically ill patients with sepsis compared with intermittent administration? Findings In this randomized clinical trial that included 607 critically ill patients with sepsis or septic shock, continuous administration of meropenem, compared with intermittent administration, did not significantly decrease the composite of all-cause mortality and the emergence of pan-resistant bacteria or extremely drug resistant at day 28 (47% vs 49%, respectively). Meaning Continuous administration of meropenem, compared with intermittent administration, does not improve clinically relevant outcomes in critically ill patients with sepsis. |
Importance
Meropenem is a widely prescribed β-lactam antibiotic. Meropenem shows maximal pharmacodynamic efficacy when administered by continuous infusion to deliver constant drug levels above the minimum inhibitory concentration. Compared with intermittent administration, continuous administration of meropenem may improve clinical outcomes.
Aim
To determine whether continuous administration of meropenem reduces a combination of mortality and emergence of pan- or extensively drug-resistant bacteria compared with intermittent administration in critically ill patients with sepsis.
Design, environment and participants
A randomized, double-blind clinical trial enrolling critically ill patients with sepsis or septic shock who had been prescribed meropenem by their treating physicians in 31 intensive care units of 26 hospitals in 4 countries (Croatia, Italy, Kazakhstan, and Russia). Patients were enrolled between June 5, 2018 and August 9, 2022, and the final 90-day follow-up was completed in November 2022.
Interventions
Patients were randomly assigned to receive an equal dose of the antibiotic meropenem by continuous administration (n = 303) or intermittent administration (n = 304).
Main results and measures
The primary outcome was a composite of all-cause mortality and emergence of pan- or extensively drug-resistant bacteria at day 28.
There were 4 secondary outcomes , including days alive and free of antibiotics at day 28, days alive and free from the intensive care unit at day 28, and all-cause mortality at day 90. Seizures, allergic reactions, and Mortality was recorded as adverse events.
Results
All 607 patients (mean age, 64 [SD, 15] years; 203 were women [33%]) were included in the 28-day primary outcome measurement and completed the 90-day mortality follow-up. The majority (369 patients, 61%) presented septic shock.
The median time from hospital admission to randomization was 9 days (IQR, 3-17 days) and the median duration of meropenem treatment was 11 days (IQR, 6-17 days). Only 1 crossover event was recorded.
The primary outcome occurred in 142 patients (47%) in the continuous group and 149 patients (49%) in the intermittent group (relative risk, 0.96 [95% CI, 0.81-1.13]. ], P = .60).
Of the 4 secondary outcomes, none were statistically significant. No adverse events of seizures or allergic reactions related to the study drug were reported.
At 90 days, mortality was 42% in both the continuous administration group (127 of 303 patients) and the intermittent administration group (127 of 304 patients).
Discussion
In this double-blind international RCT of critically ill patients with sepsis, there were no significant differences in the composite outcome of all-cause mortality and emergence of drug-resistant or extremely drug-resistant bacteria at 28 days for continuous administration versus intermittent administration. of meropenem. No significant differences were observed for any of the 4 secondary outcomes or for the individual elements of the composite primary outcome.
The results of the current study suggest that continuous administration of meropenem does not improve clinically relevant outcomes in critically ill patients with sepsis, including long-term mortality.
Previous studies found short-term survival benefits, while 1 study reporting 90-day mortality showed a nonsignificant difference (26% in the continuous group vs. 28% in the intermittent group, P = 0.67), 40 which is consistent with the current study (42% in both groups, p = 0.97).
The experts further suggested that the method of administering meropenem should take into account other factors, such as feasibility, availability of an intravenous line, issues with drug stability, costs, and logistical issues. 45Furthermore, the experts indicated that no specific subgroups could be identified in which continuous administration could be the target of future RCTs.
Conclusions and relevance
In critically ill patients with sepsis, compared with intermittent administration, continuous administration of meropenem did not improve the composite outcome of mortality and emergence of pan- or extensively drug-resistant bacteria at day 28.
Trial registration: ClinicalTrials.gov Identifier: NCT03452839















