In this article, we highlight the current understanding of the effect of corticosteroid therapy in severe COVID-19.
| 1. Evidence of effectiveness from randomized controlled trials in patients hospitalized with COVID-19 |
The RECOVERY randomized controlled trial (RCT) demonstrated that dexamethasone (6 mg daily for 10 days) in hospitalized patients with COVID-19 reduced (i) 28-day mortality (rate ratio 0.83, confidence interval [ 95% CI, 0.75-0.93), (ii) duration of hospitalization and (iii) progression to invasive mechanical ventilation.
The greatest reduction in mortality was seen in those receiving supplemental oxygen or invasive mechanical ventilation; no improvement was seen in those without respiratory support [1]. A prospective meta-analysis of 7 RCTs further confirmed the benefit of corticosteroid therapy in reducing mortality in critically ill patients with COVID-19 (summary odds ratio [OR], 0.66; 95% CI, 0.53). -0.82). This is the best direct evidence supporting corticosteroid therapy in severe COVID-19.
| 2. Evidence of effectiveness in non-viral acute respiratory distress syndrome (ARDS) |
A recent Spanish RCT (n = 277) of patients with moderate to severe ARDS found that dexamethasone (20 mg and 10 mg for 5 days each), compared with placebo, was associated with more days without mechanical ventilation and fewer mortality. An updated meta-analysis of ten RCTs shows that corticosteroid treatment initiated before day 14 of ARDS was associated with a significant reduction in the duration of mechanical ventilation and in-hospital mortality (risk ratio (RR) 0.67, 95% CI). %: 0.52 to 0.87).
| 3. Evidence of effectiveness in community-acquired pneumonia |
Several systematic reviews demonstrated that in patients hospitalized with community-acquired pneumonia (CAP), corticosteroid therapy was associated with a reduction in mortality, length of stay, and time to clinical stability.
| 4. Dysregulated immune response in COVID-19 |
Biological improvement was associated with accelerated resolution of the disease.
Corticosteroid therapy aims to support the central regulatory function of activated glucocorticoid receptor α (GC-GRα) during disease development and resolution. The dysregulated immune response observed in COVID-19 is qualitatively similar to that in multifactorial ARDS. In patients with severe COVID-19, glucocorticoid receptor expression in bronchoalveolar lavage myeloid cells is negatively associated with pulmonary neutrophilic inflammation, netosis, and symptom severity.
Translational research in ARDS patients randomized to methylprednisolone has demonstrated the ability of corticosteroid therapy to rescue cellular concentrations and functions of activated GC-GRα, leading to downregulation of markers of inflammation, coagulation and fibroproliferation activated by systemic and pulmonary nuclear factor κB. Biological improvement was associated with accelerated resolution of the disease. More work is needed to understand the effect of corticosteroid therapy on the immune response to COVID-19.
| 5. CT and pathology findings |
The computed tomographic findings of the ground-glass appearance and histopathologic features ( post-mortem studies ) of diffuse alveolar damage and acute fibrinous and organizing pneumonia are consistent with corticosteroid-responsive inflammatory lung diseases.
| 6. The role of the hypothalamic - pituitary - adrenal axis |
Evidence from severe acute respiratory syndrome (SARS) studies suggests that SARS-CoV-2 infection is associated with an impaired cortisol stress response (Fig. 1).
| 7. Microthrombi and coagulopathy |
The pathogenesis of COVID-19 disease appears to be induced by dysregulated systemic and pulmonary inflammation, along with endothelial injury, hypercoagulability, and thrombosis.
Platelet and fibrin thrombus formation in small arterial vessels is commonly observed on post-mortem examination of the lungs of COVID-19 patients. Emerging data indicate that hypercoagulability in COVID-19 is induced by the deregulated release of neutrophil extracellular traps (NETs). In an equine experimental model, dexamethasone reduced NET formation, which may contribute to the observed benefit of corticosteroid therapy in COVID-19.
| 8. Acceptable security profile |
Short-term corticosteroid therapy (up to 4 weeks) in patients with life-threatening systemic inflammation is well tolerated. Data from systematic reviews in CAP and ARDS showed that corticosteroid therapy was associated with transient hyperglycemia but did not increase the frequency of gastrointestinal bleeding, neuromuscular weakness, or nosocomial infections. Hyperglycemia did not affect the result.
Recent data have provided evidence that GRα is essential for the activation and reinforcement of innate immunity and when applied correctly (i.e., appropriate duration of corticosteroid administration) is associated with restoration of anatomy and function. of the affected tissues, together with a parallel support of adaptive immunity. Downregulation of systemic and pulmonary inflammation associated with corticosteroid treatment could reduce the risk of developing nosocomial infections by:
(i) Reduce the duration of mechanical ventilation.
(ii) Achieve a less favorable inflammatory environment for the intra- and extracellular growth of bacterial pathogens frequently found in ARDS (Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter sps.).
(iii) Improve phagocytic neutrophil function dependent on opsonization and intracellular destruction.
| 9. Long-term result |
In RCTs of patients with ARDS, corticosteroid therapy was associated with a survival benefit that persisted up to one year after hospital discharge (limit of measurement). An extensive literature suggests that proinflammatory cytokines influence the brain and may be involved in the pathogenesis of depression and post-traumatic stress disorder (PTSD). Data from five small RCTs (n = 292) suggest that longer duration of corticosteroid therapy may be associated with lower anxiety scores and better PTSD symptomatology.
The corticosteroid therapy-associated reduction in the duration of mechanical ventilation and sedation may also have a positive impact on long-term PTSD symptoms and cognitive function.
| 10. Scalability |
Low cost and wide availability make corticosteroid therapy easily equitable and available globally in different income settings.
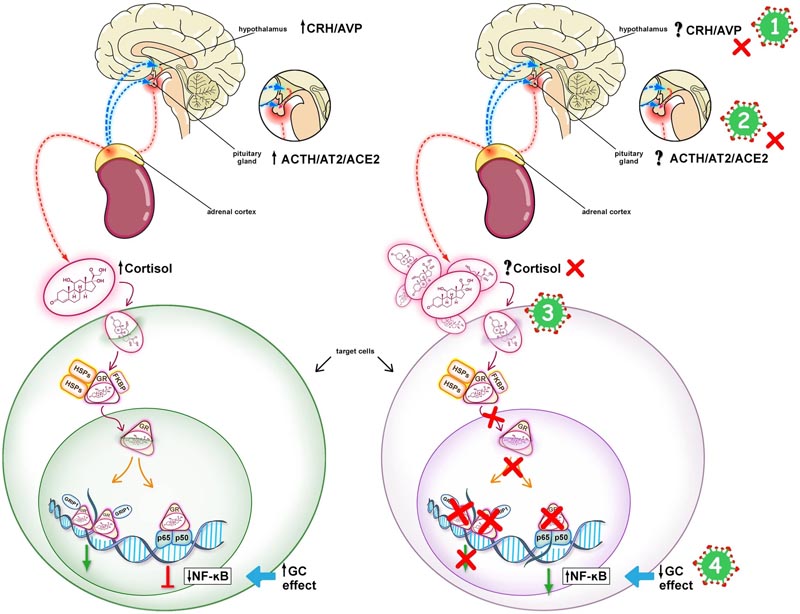
Hypothalamic-pituitary-adrenal (HPA) response in COVID-19. The left panel shows an intact HPA axis response to stress leading to downregulation of NF-kB. The right panel shows the mechanisms potentially involved in an impaired HPA axis response to stress in COVID-19. COVID-19 may be associated with CIRCI, as a result of inhibition of the hypothalamic-pituitary-adrenal (HPA) axis at any or all of its levels, as shown by the red Xs: (1) Hypothalamus (CRH/AVP), ( 2) pituitary gland (ACTH/AT2/ACE2), (3) adrenal cortex (cortisol), and (4) target tissues (glucocorticoid receptor signaling system). In CIRCI, target tissue resistance to glucocorticoids can occur even in the presence of elevated levels of circulating cortisol. SARS-CoV-2 has an epitope that binds to the ACE2 enzyme, facilitating entry into host cells, which could influence the function of the HPA axis at the level (2). SARS-CoV-2 may have an ACTH mimicking peptide that could act as an agonist or antagonist at the ACTH receptor level (3). Long-term glucocorticoid treatment is indicated when cortisol production and/or target tissue sensitivity to cortisol is compromised (Online Supplement for supplemental references). CIRCI Critical illness-related corticosteroid insufficiency, corticotropin-releasing hormone CRH, AVP arginine-vasopressin, angiotensin-converting enzyme 2 ACE2, angiotensin 2 AT2, corticotropin ACTH, glucocorticoid receptor GR - alpha, heat protein HSP, immunofilvin co-agent shock FKBP, GRIP1 p65/p50 nuclear factor (NF)-kB
Recent data on corticosteroid therapy represents a milestone in the management of COVID-19, while many questions remain. Efforts for global implementation of corticosteroid treatment protocols should be supported by data on generalizability of effect observed in randomized controlled trials in patient subgroups, different populations, and resource settings.
More data are needed to evaluate the impact of corticosteroid type, time of initiation, dose, mode of administration, duration, and dose tapering on outcome. And also to (i) identify modalities to adjust the dose and duration of treatment based on laboratory parameters of oxygenation and inflammation, and (ii) evaluate the impact of co-interventions aimed at improving response to corticosteroid therapy .
Currently, limited data are available on the impact of corticosteroid therapy on SARS CoV-2 replication and on the potential benefits of concomitant antiviral treatment. The interaction between corticosteroid therapy and other COVID-19 therapies, such as interferons (IFNs) and anticoagulation, should be explored.
|
















