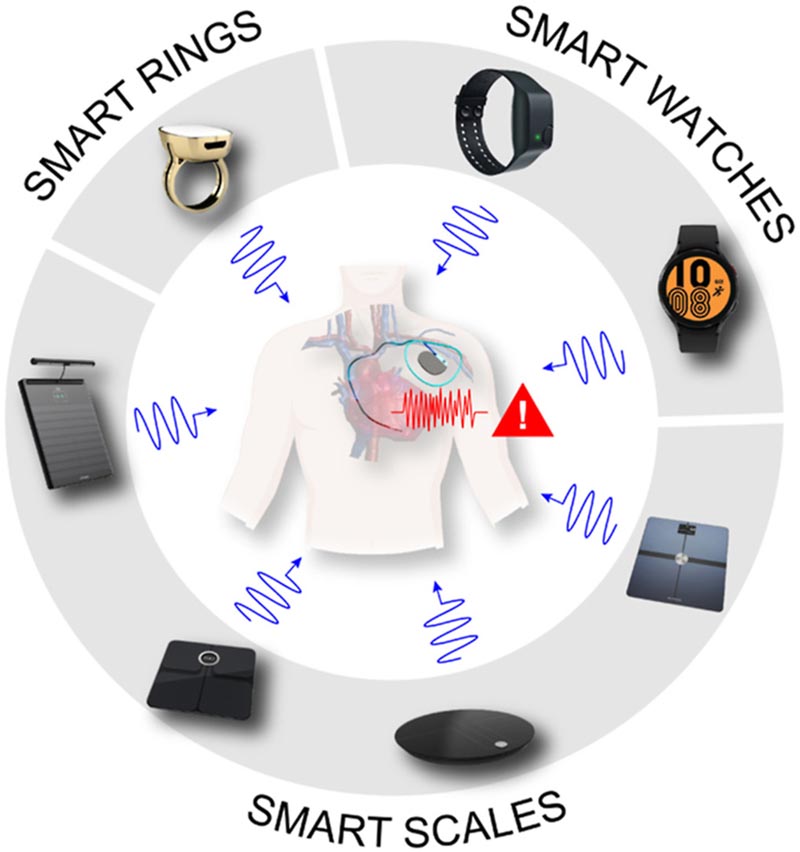
Summary Background Smart scales, smart watches, and smart rings with bioimpedance technology can create interference in patients with cardiac implantable electronic devices (CIEDs). Goals The purpose of this study was to determine interference in cardiac implantable electronic devices (CIEDs) with simulations and laboratory tests, and compare the results with the maximum values defined in the ISO 14117 electromagnetic interference standard for these devices. Methods Interference in pacemaker electrodes was determined by simulations in a male and female computable model . A laboratory evaluation of representative CIEDs from 3 different manufacturers, as specified in ISO 14117, was also performed. Results The simulations showed evidence of interference with voltage values exceeding the threshold values defined in the ISO 14117 standard. The level of interference varied with the frequency and amplitude of the bioimpedance signal, and between male and female models. The level of interference generated with the smart scale and smart ring simulations was lower than with smart watches. Across device manufacturers, generators demonstrated susceptibility to oversensing and inhibition of stimulation at different signal amplitudes and frequencies. Conclusions This study evaluated the safety of smart scales, smart watches, and smart rings with bioimpedance technology through simulation and testing. Our results indicate that these consumer electronic devices could interfere in patients with CIED . The present findings do not recommend the use of these devices in this population due to potential interference. |
Comments
Certain wearable devices have the potential to interfere with pacemakers and other implanted cardiac electronic devices, according to a new study in Heart Rhythm .
In recent years, wearable devices such as smart watches and rings, as well as smart scales, have become ubiquitous: "must-haves" for health-conscious people to self-monitor heart rate, blood pressure, and other vital signs. Despite obvious benefits, certain fitness and wellness trackers could also pose serious risks to people with cardiac implantable electronic devices (CIEDs), such as pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices. , reports a new study published in Heart Rhythm , the official journal of the Heart Rhythm Society, the Cardiac Electrophysiology Society, and the Pediatric & Congenital Electrophysiology Society, published by Elsevier.
Safety evaluation of smart scales, smart watches and smart rings with bioimpedance technology shows evidence of potential interference with cardiac implantable electronic devices
The researchers evaluated the performance of CRT devices from three leading manufacturers while applying the electrical current used during bioimpedance detection. Bioimpedance sensing is a technology that emits a very small, imperceptible current of electricity (measured in microamperes) into the body. The electrical current flows through the body and the sensor measures the response to determine the person’s body composition (i.e., skeletal muscle mass or fat mass), stress level, or vital signs such as respiratory rate.
“Bioimpedance detection generated electrical interference that exceeded the guidelines accepted by the Food and Drug Administration and interfered with the proper functioning of the CIED,” explained principal investigator Benjamin Sanchez Terrones, PhD, Department of Electrical and Computer Engineering at the University of Utah, Salt Lake City, UT. The US stressed that the results, determined through careful simulations and laboratory tests, do not convey an immediate or clear risk to patients wearing the trackers, but noted that the different levels emitted could cause disruptions in the rhythm or unnecessary shocks to the heart. Dr. Sánchez added, "Our findings call for future clinical studies examining patients with CIED and wearable devices."
The interaction between household appliances in general and, more recently, smartphones, with CIEDs has been the subject of study within the scientific community in recent years. Almost all, if not all, implantable cardiac devices already warn patients about the potential for interference with a variety of electronic devices due to magnetic fields, for example, carrying a cell phone in a chest pocket near a pacemaker. The rise of wearable health technology has grown rapidly in recent years, blurring the line between medical and consumer devices. Until this study, objective evaluation to ensure safety has not kept pace with new and exciting devices.
“Our research is the first to study devices that employ bioimpedance sensing technology and to uncover potential interference issues with CIEDs, such as CRT devices. We need to test in a larger cohort of devices and in patients with these devices. Collaborative research between researchers and industry would be useful to keep patients safe,” said Dr. Sánchez Terrones.
Final message This study provides baseline data to evaluate the safety of smart scales, smart watches, and smart rings with bioimpedance technology in CIEDs. The methodology and results presented represent the most comprehensive technical analysis currently available on the level of interference induced by bioimpedance devices in CIEDs using the FDA-accepted ISO 14117 standard as a reference. Our results suggest that consumer electronic devices with bioimpedance technology could induce electrical voltage higher than the maximum values of the ISO 14117 standard for CIEDs. These serious adverse events can occur without warning in patients with CIED and have the potential to interfere with life-saving therapy when these patients undergo bioimpedance measurements using smart scales, wearable smart watches, and smart rings. The present findings do not recommend the use of these devices with bioimpedance technology in this population due to possible electrical interference. These results also require a review and update of the ISO 14117 standard to define new specific tests for devices with bioimpedance technology. Future clinical studies should take our findings into account and include more confirmatory simulation and laboratory studies considering worst-case conditions to determine the clinical relevance and corroborate the safety of smart scales, wearable smartwatches, and rings. smart with bioimpedance technology in patients with CIED. |