Summary Recent clinical trials in people with type 2 diabetes have demonstrated beneficial actions on cardiac and renal outcomes after treatment with GLP-1 receptor agonists (GLP-1RA). In part, these actions are consistent with improved glucose control and significant weight loss. But GLP-1RAs may also have additional benefits by improving postprandial dysmetabolism. In diabetes, dysregulated postprandial nutrient functions trigger inflammation, oxidative stress, endothelial dysfunction, thrombogenicity, and endotoxemia ; alterations in hormonal levels; and modulation of cardiac output and regional blood and lymphatic flow. In this perspective, we explore the actions of GLP-1RAs in the postprandial state and their potential role in the target organ benefits observed in recent trials. |
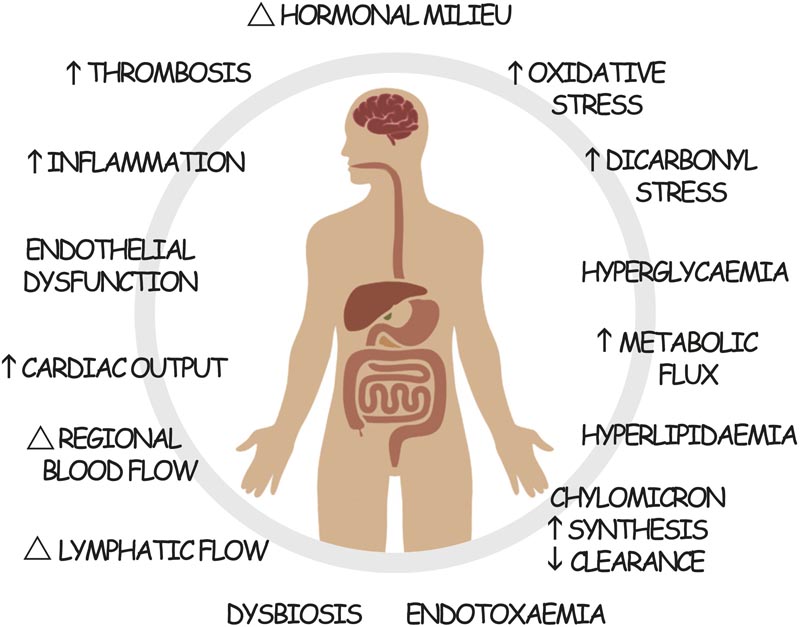
Postprandial dysmetabolism contributes to the development and progression of cardiovascular and renal disease in type 2 diabetes. Each of these pathogenic mechanisms can be modified by treatment with GLP-1RA.
Ninety years ago, LaBarre first described "incretins" contained in extracts of duodenal mucosa that reduced glucose levels in dogs, supposedly by increasing insulin secretion, leading to its name (from intestinal insulin secretion). ).
Incretins were conceived as endocrine equivalents of secretin, a hormone released by the intestine identified 30 years earlier, with postprandial actions to regulate pancreatic fluid secretion and gastric accommodation. The greater release of insulin (incretin) in response to an oral glucose challenge compared to the same glucose load administered intravenously later became known as the "incretin effect" , and glucagon-like peptide-1 (GLP-1) 1) was later identified as its key insulinotropic mediator.
In 1993 it was shown that an infusion of GLP-1 could reduce plasma glucose levels in people with type 2 diabetes (T2D). And more recently, GLP-1 analogues (exendin-based) and long-acting versions of human GLP-1 have been developed for clinical use in T2D and obesity.
GLP-1RAs are now widely used as glucose-lowering agents in people with T2D, where the addition of a GLP-1RA to standard treatment consistently reduces HbA1c by approximately 1%.5 In comparative clinical trials, GLP-1RAs achieve similar or better reductions in HbA1c, fasting glucose, and postprandial glucose control than titrated basal insulin or basal bolus therapy.
Additionally, GLP-1RAs have a lower handling burden than insulin, associated with a reduced risk of hypoglycemia , lack of weight gain, and reduced requirement for glucose self-monitoring or consistency in dietary carbohydrate intake. For these reasons, and the target organ benefits, GLP-1RAs are now recommended as the preferred injectable agent for the treatment of T2D over insulin in most cases.
More recently, GLP-1RAs have also been shown to be highly effective in inducing weight loss , primarily through improved appetite control and satiety and subsequent reduction in energy consumption. While modest weight loss was observed in trials conducted in people with T2D, recent studies using high doses of GLP-1RA in people with obesity have now demonstrated highly significant changes in body weight as a primary outcome. For example, a recent study showed that almost 40% of participants treated with semaglutide once a week lost more than 20% of their body weight.
However, the clinical actions of GLP-1RAs appear to be much broader than glucose lowering or weight loss induction. In particular, recent large trials in people with T2D have shown unexpected benefits on different cardiovascular and renal outcomes , in addition to its obvious actions in improving glucose control and reducing body weight. In this perspective, we propose that the unique actions of GLP-1RA on postprandial dysmetabolism associated with T2D (Figure 1) may partly confer these additive benefits.
Conclusions
GLP-1RAs improve MACE outcomes in people with T2D with established CVD or high cardiovascular risk and are now widely recommended as an adjunct to standard care in this setting.
In patients with T2D and CKD, GLP-1RAs are also recommended, in part due to the high cardiovascular risk associated with CKD and the greater absolute benefit of treatment with GLP-1RAs, as well as emerging evidence of actions renoprotectors that are currently being investigated. Tested in large studies specific to kidney outcomes. In each case, the benefits of GLP-1RAs appear to largely be conferred by substantially better glucose control and significant weight loss, which are recognized as key pillars for the multifactorial management of T2D.
Furthermore, those agents that achieve greater glucose reduction and weight loss also appear to have greater cardiovascular and renal benefits. But at the same time, to the extent that these actions reflect the potency of the agent, GLP-1RAs that are more effective at lowering glucose or promoting weight loss may logically also be more effective at improving cardiovascular or renal due to shared mechanism of action (e.g., GLP-1R activation).
However, neither HbA1c reduction nor weight loss appear to fully explain the observed target organ benefits. We speculate that blunt instruments used to measure diabetes control (e.g., HbA1c, fasting lipids, body weight, office blood pressure, etc.) fail to comprehensively capture the full activity of diabetes therapy. GLP-1RA. In particular, we hypothesize that the (unmeasured) actions of GLP-1RAs in modulating postprandial dysmetabolism in T2D (Figure 1) may contribute to the apparent additive benefits of GLP-1RAs over conventional therapies. of standard care observed. By attenuating dysregulated postprandial glucose and lipid spikes and the resulting flares of inflammation, oxidative stress, endothelial dysfunction, thrombogenicity, endotoxemia, and altered regional blood and lymphatic flow, the development and progression of cardiovascular and renal disease can be further slowed.
The modern unhealthy lifestyle means that much of our waking day is now spent in a postprandial state.
During this period, processed foods rich in fats and/or carbohydrates and low in fiber are digested. The cost of this lifestyle is potentially our health, including higher rates of obesity, T2D, ASCVD, and CKD. The missing link in managing these conditions is focusing on the immediate effects of our foods. This is not a new idea. In fact, atherosclerosis was proposed as a postprandial phenomenon in 1979.
The effects of postprandial metabolism likely support the observed health benefits of dietary macronutrient modification, beyond weight control. Furthermore, the actions of dietary fiber and micronutrients, such as polyphenols, antioxidants, and even ethanol, on postprandial metabolism may also partly explain some of their cardiovascular and/or renal benefits. Therefore, it is plausible that, beyond the cumulative effects on HbA1c or body weight, direct modulation of the postprandial state by GLP-1RAs also plays a role in the benefits observed after treatment with these agents. . Furthermore, central signaling activated by GLP-1RAs not only modulates appetite and calorie intake, but also significantly influences food choice or preference , to reduce the intake of high-fat and high-energy foods.
For example, tirzepatide reduces preference for high-fat, high-calorie foods in a GLP-1R-dependent manner. At the same time, exenatide increased preferences for low-calorie foods as measured by brain activation after viewing images of different foods. GLP-1RA treatment is able to reduce food cravings and snacking between meals and also modulates hedonic food intake. The improvement in the postprandial environment achieved indirectly by the preference and selection of healthier foods with less energy density may be as important for cardiovascular and renal risk as any direct action of GLP-1RAs on the same risks, with which There is an obvious synergy.
The development and evolution of GLP-1RAs continues to revolutionize the management of T2D, with newer, more potent agonists that profoundly lower glucose levels and result in substantial losses of excess body fat being increasingly identified. . The pleiotropic actions of GLP-1RAs in T2D, although significant, likely pale in comparison to the agents’ ability to put T2D or obesity into “remission ,” and the side effects this will have on cardiovascular and renal health. . However, improving the understanding of these pathways will also likely lead to new opportunities to improve cardiovascular and renal activity, beyond metabolism, through more potent receptor agonism, biased signaling, and hybrid multireceptor approaches to better regulate the postprandial state. The outstanding clinical activity already observed with tirzepatide , a potent and biased agonist of both GLP-1R and GIP-R, is only a preview of the therapeutic revolution to come.
















