The year 2022 has been an exciting year in heart failure (HF). This brief report highlights some of the most provocative and impactful articles in the field.
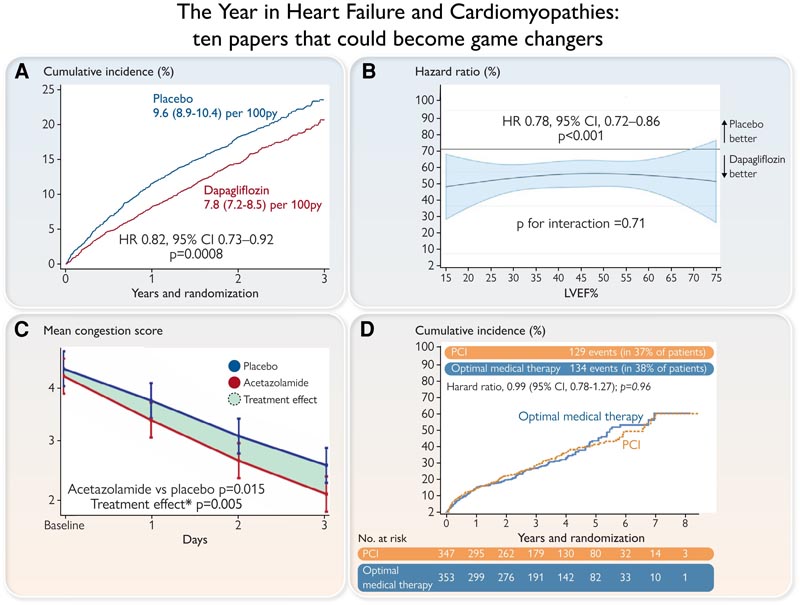
Panel A. DELIVER primary endpoint [cardiovascular (CV) death or hospitalization for heart failure (HF)] in the entire population (left) and in the population with LVEF <60%. Panel B. A pooled analysis of patients enrolled in the DAPA-HF and DELIVER trials reveals a consistent benefit of dapagliflozin on the primary endpoint (CV death or HF hospitalization) across the spectrum of LVEF, with no signs of attenuation of the effect in the highest range of LVEF. Panel C. Effect of acetazolamide on congestion in the ADVOR trial. From Day 1 onwards, use of acetazolamide in addition to regular loop diuretics resulted in accelerated decongestion. Panel D. Kaplan-Meier estimates of all-cause mortality or hospitalization for HF for patients receiving PCI or optimal medical treatment in the REVIVED trial. LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
| Sodium-glucose cotransporter 2 inhibitors |
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are becoming one of the main treatments for patients with cardiorenal disease. Some doubts remained, for example, whether SGLT2 inhibitors were effective in patients with acute HF (AHF), or in HF with a left ventricular ejection fraction (LVEF) > 40%, or in patients with improved LVEF.
The Study to Test the Effect of Empagliflozin in patients Who Are in Hospital for Acute Heart Failure (EMPULSE) trial enrolled 530 patients with de novo acute heart failure or decompensated HF to receive empagliflozin 10 mg once daily or placebo. Patients were randomized in the hospital, when they were clinically stabilized, and were treated for up to 90 days. More patients treated with empagliflozin had clinical benefits compared to placebo. Mortality and readmissions due to HF were also reduced.
The Dapagliflozin Evaluation to Improve the LIVEs of patients with PReserved Ejection Fraction Heart Failure (DELIVER) study was a double-blind randomized clinical trial in 6263 patients with chronic symptomatic heart failure, LVEF > 40% and elevated natriuretic peptides that compared the effect of dapagliflozin 10 mg once daily versus placebo, in addition to standard treatment. After 28 months of follow-up, the primary outcome [death from cardiovascular (CV) causes or hospital admissions for HF] occurred in 16.4% in the dapagliflozin group and 19.5% in the placebo group. The frequency of adverse events leading to treatment discontinuation, related to volume depletion and hypoglycemia, was similar between groups.
| Diuresis, kidney function, sodium and potassium. |
The topic of sodium restriction in HF has been discussed for a long time, and the Dietary Intervention Below 100 mmol in Heart Failure (SODIUM-HF) study was designed to evaluate whether a reduction in dietary sodium whether or not it reduces the incidence of future clinical events. SODIUM-HF recruited 806 patients with chronic heart failure receiving guideline-directed medical treatment and randomized them to receive usual care according to local guidelines or a low-sodium diet (LSD) of <100 mmol (that is, < 1500 mg/day).
Median sodium intake decreased from 2286 mg/day to 1658 mg/day in the low-sodium group and from 2119 mg/day to 2073 mg/day (1541 –2900) in the usual care group. At 12 months, CV-related hospital admission, CV-related emergency department visit, or all-cause death had occurred in 15% of patients in the LSD group and 17% in the usual care group. Therefore, a dietary intervention to reduce sodium intake does not reduce clinical events.
Patiromer is a potassium reducing agent. The Medications for the Treatment of Heart Failure (DIAMOND) trial investigated the effects of patiromer on serum potassium level and whether its use would allow the use of target doses of renin-angiotensin-aldosterone system inhibitors (RAASi) in patients with HFrEF.
A total of 1195 patients were enrolled during the run-in phase with patiromer and RAASi therapy optimization [≥recommended dose of 50% RAAS and 50 mg mineralocorticoid receptor antagonist (MRA)]; this was achieved in 878 (84.6%) of the patients who were randomized 1:1. At the end of treatment, the adjusted mean change in potassium was +0.03 mmol/L in the patiromer group and +0.13 mmol/L in the placebo group. This was accompanied by a lower risk of hyperkalemia. (>5.5 mmol/L) and fewer reductions in MRA dose.
Surprisingly, a large proportion of patients with past hyperkalemia who had their RAASi or MRA dose reduced were able to tolerate adequate doses of RAASi and/or MRA during the initial phase of the trial. In any case, patiromer allows adequate titration of RAASi and MRA in patients with hyperkalemia, although the number needed to treat to prevent difficult clinical outcomes with this strategy appears to be quite high.
Diuretic resistance is another clinical dilemma that was addressed by two interesting trials. The Acetazolamide in Acute Decompensated Heart Failure with Volume Overload (ADVOR) study evaluated whether acetazolamide , a carbonic anhydrase inhibitor, reduces sodium reabsorption in the proximal tubule, in addition to loop diuretics in patients with AHF; 519 patients with AHF and clinical signs of volume overload and an NT-proBNP level of more than 1000 pg/mL were randomized to receive intravenous acetazolamide (500 mg once daily) or placebo added to standardized intravenous loop diuretics.
Successful decongestion was achieved more frequently in the acetazolamide group compared to the placebo group.
Acetazolamide treatment was associated with greater cumulative diuresis and natriuresis, findings consistent with better diuretic efficacy. The incidence of worsening renal function, hypokalemia, hypotension, and adverse events was similar in the two groups. These data are likely to change the standard diuretic regimen in AHF.
The safety and efficacy of combining loop diuretics with thiazide-type diuretics in patients with decompensated heart failure ( CLOROTIC trial ) evaluated whether the addition of hydrochlorothiazide (HCT) to intravenous furosemide is a safe and effective strategy to improve diuretic response in patients with AHF. In total, 230 patients (48% female, 83 years old) were randomized to HCT or placebo; those on HCT lost more weight at 72 h but there were no significant differences in patient-reported dyspnea. Rates of mortality or rehospitalization for heart failure were similar between HCT and placebo. HCT patients more frequently had a significant increase in creatinine.
| Cardiomyopathies, revascularization, heart failure |
One article addressed the long-standing dispute over whether or not patients with ischemic cardiomyopathy can benefit from revascularization by percutaneous coronary intervention (PCI), compared with optimal medical therapy (OMT) (i.e., adjusted drug and device therapy). individually for IC).
Patients with an LVEF of 35% or less, extensive coronary artery disease amenable to PCI, and demonstrable myocardial viability were randomized to receive PCI plus OMT (PCI group) or OMT alone. Totally, 347 were assigned to the PCI group and 353 to the OMT group. Over 41 months, a primary event (death from any cause or hospitalization for HF) occurred in 37.2% of the PCI group and 38.0% of the OMT group. Therefore, PCI revascularization has no benefit in these patients other than medical therapy.
Finally, two interesting articles addressed how drug titration can be managed in patients with HF .
- Accelerated and personalized therapy for heart failure with reduced ejection fraction . Eur Heart J 2022
- Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomized, trial. Lancet 2022
Until recently, guidelines recommended starting treatment in heart failure patients with a slow, controlled escalation of individual drug classes. However, the most recent guidelines state that four classes of drugs should be titrated on a faster schedule; Despite this, the order and speed of titration remained unaddressed.
Overall, these two trials provide strong support for accelerated guideline-directed titration of drug treatment, while the order of medications administered need not be based on historical background.
















