Small interfering RNA to reduce lipoprotein (a) in cardiovascular disease
Summary
Background
Lipoprotein (a) is a presumed risk factor for atherosclerotic cardiovascular disease. Olpasiran is a small interfering RNA that reduces lipoprotein (a) synthesis in the liver.
Methods
We conducted a randomized, double-blind, placebo-controlled, dose-finding trial in patients with established atherosclerotic cardiovascular disease and a lipoprotein (a) concentration of more than 150 nmol per liter. Patients were randomly assigned to receive one of four doses of olpasiran (10 mg every 12 weeks, 75 mg every 12 weeks, 225 mg every 12 weeks, or 225 mg every 24 weeks) or a matching placebo, administered subcutaneously.
The primary endpoint was the percentage change in lipoprotein (a) concentration from baseline to week 36 (reported as the placebo-adjusted mean percentage change). Safety was also evaluated.
Results
Among the 281 patients enrolled, the mean lipoprotein (a) concentration at baseline was 260.3 nmol per liter, and the mean low-density lipoprotein cholesterol concentration was 67.5 mg per deciliter.
At baseline, 88% of patients were receiving treatment with statins, 52% with ezetimibe, and 23% with a proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitor. At 36 weeks , lipoprotein(a) concentration had increased by a mean of 3.6% in the placebo group, while olpasiran treatment had significantly and substantially reduced lipoprotein(a) concentration in a weight-dependent manner. dose, resulting in a placebo-adjusted mean. percentage changes of -70.5% with the 10 mg dose, -97.4% with the 75 mg dose, -101.1% with the 225 mg dose given every 12 weeks, and -100.5% with the 225 mg dose given every 24 weeks (P<0.001 for all comparisons to baseline).
The overall incidence of adverse events was similar between trial groups. The most common adverse events related to olpasiran were injection site reactions, primarily pain.
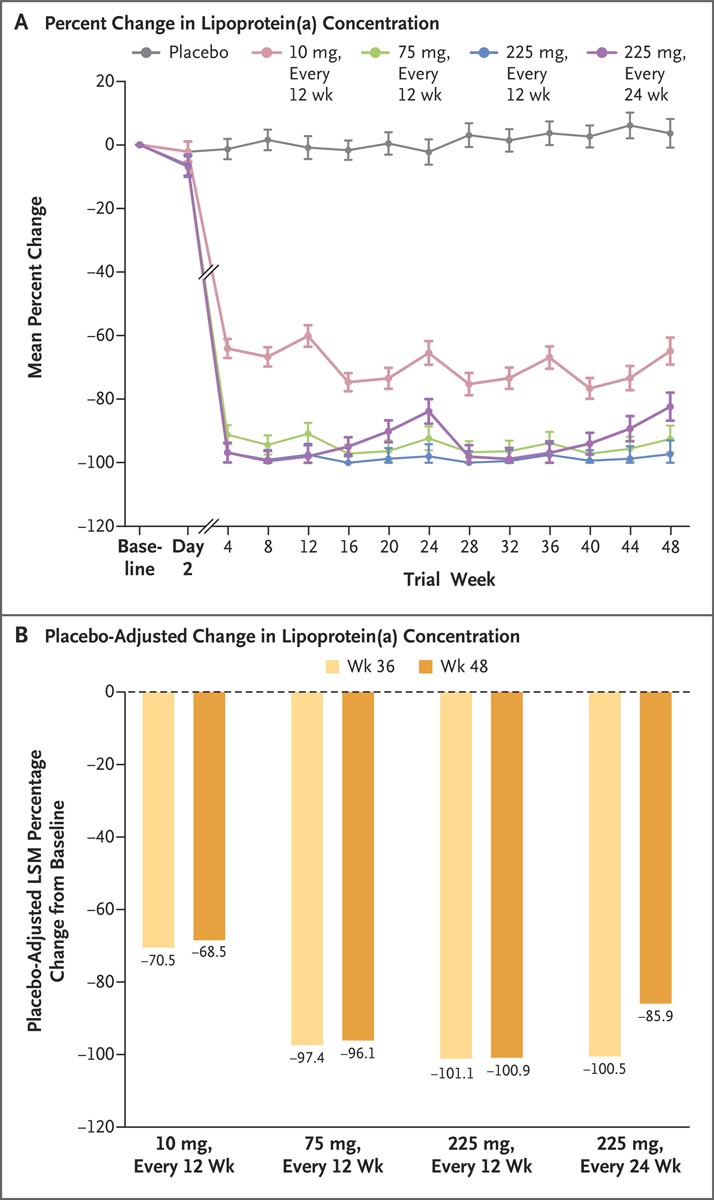
Percent change in lipoprotein(a) concentration and placebo-adjusted mean percent change from baseline in lipoprotein(a) concentration with olpasiran at weeks 36 and 48. Panel A shows the mean percent change in lipoprotein(a) concentration lipoprotein (a) over time according to test group. Bars indicate 95% confidence intervals. Panel B shows the placebo-adjusted least squares mean (LSM) percent change from baseline in lipoprotein (a) concentration for the four olpasiran dose groups at weeks 36 and 48.
Conclusions Olpasiran therapy significantly reduced lipoprotein (a) concentrations in patients with established atherosclerotic cardiovascular disease. Larger and longer trials will be needed to determine the effect of olpasiran therapy on cardiovascular disease. |
(Funded by Amgen; OCEAN[a]-DOSE ClinicalTrials.gov number, NCT04270760. opens in new tab.)
Comments
Lipoprotein (a) is a special type of bad cholesterol that is thought to contribute to heart disease, but there are no approved drug therapies to lower its concentration in the bloodstream. Olpasiran is an investigational drug that reduces the concentration of lipoprotein (a) by degrading the RNA that codes for a protein that is an essential part of the molecule. Researchers at Brigham and Women’s Hospital, a founding member of the Mass General Brigham healthcare system, conducted a Phase 2, randomized, placebo-controlled clinical trial of olpasiran in patients with established cardiovascular disease to evaluate its safety and tolerability and identify an optimal dose. of olpasiran to reduce lipoprotein(a) levels.
The trial included 227 patients who received one of four doses of olpasiran and 54 who received a placebo. They found that patients receiving higher doses of olpasiran had a more than 95% reduction in lipoprotein (a) over 36 weeks compared to placebo. The treatment was not associated with serious side effects, other than occasional swelling at the injection site and related mild reactions.
"The results of this study show that a marked and sustained reduction in lipoprotein(a) is possible through RNA interference using olpasiran," said lead author Michelle O’Donoghue, MD, MPH, Cardiovascular Division, Brigham and Women’s Hospital. “These findings set the stage for a much larger phase 3 trial to definitively evaluate whether lowering lipoprotein(a) translates into better outcomes. “Olpasiran is a very promising therapy for people with high levels of lipoprotein (a) who currently have no effective therapies to reduce its concentration.”
















