Polypill reduces cardiovascular events in heart attack patients compared to usual care
SECURE essay presented in a Hot Line Session at the ESC Congress 2022
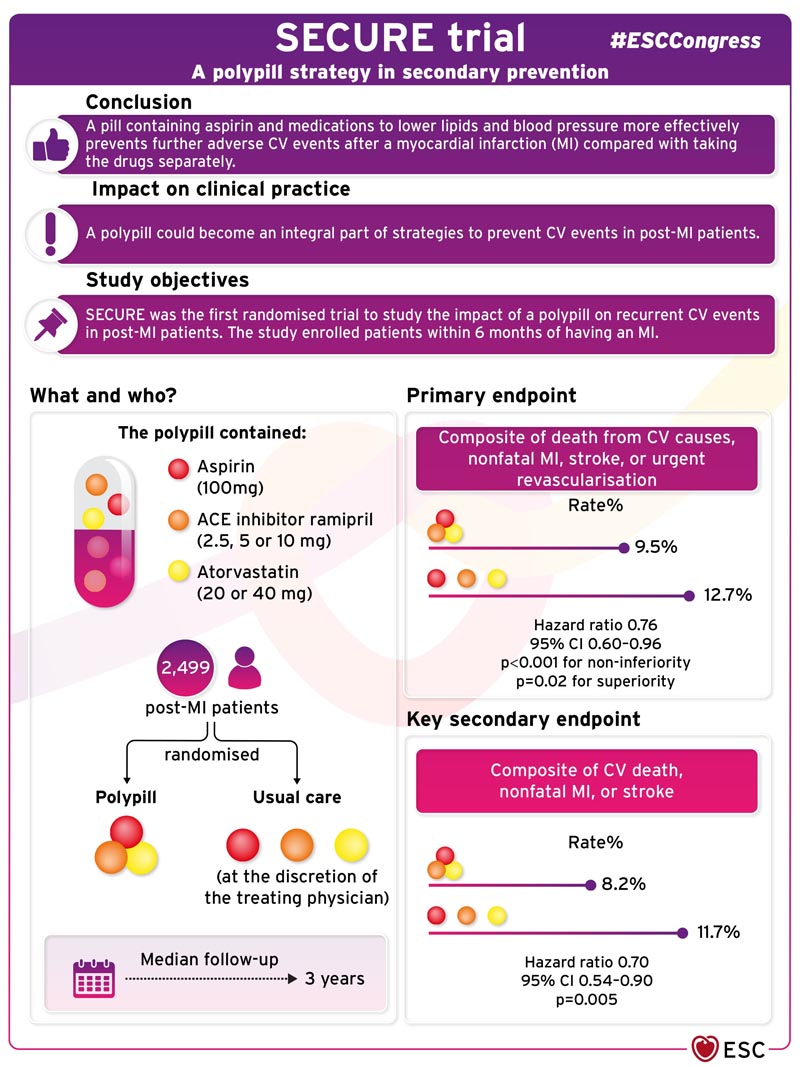
Background A polypill that includes key medications associated with better outcomes (aspirin, angiotensin-converting enzyme [ACE] inhibitor, and statin) has been proposed as a simple approach for secondary prevention of cardiovascular death and complications after myocardial infarction. Methods In this phase 3 randomized controlled clinical trial, we assigned patients with myocardial infarction within the previous 6 months to a polypill-based strategy or usual care. Polypill treatment consisted of aspirin (100 mg), ramipril (2.5, 5, or 10 mg), and atorvastatin (20 or 40 mg). The primary composite outcome was cardiovascular death, nonfatal type 1 myocardial infarction, nonfatal ischemic stroke, or urgent revascularization. The key secondary endpoint was a composite of cardiovascular death, nonfatal type 1 myocardial infarction, or nonfatal ischemic stroke. Results A total of 2499 patients were randomized and followed for a median of 36 months. A primary outcome event occurred in 118 of 1237 patients (9.5%) in the polypill group and 156 of 1229 (12.7%) in the usual care group (hazard ratio, 0.76; range 95% confidence interval [CI], 0.60 to 0.96; P=0.02). A key secondary outcome event occurred in 101 patients (8.2%) in the polypill group and 144 (11.7%) in the usual care group (hazard ratio, 0.70; 95% CI). , 0.54 to 0.90; P = 0.005). Results were consistent across prespecified subgroups. Patient-reported medication adherence was higher in the polypill group than in the usual care group. Adverse events were similar between groups. Conclusions Treatment with a polypill containing aspirin, ramipril, and atorvastatin within 6 months of myocardial infarction resulted in a significantly lower risk of major adverse cardiovascular events than usual care. (Funded by the European Union Horizon 2020; SECURE ClinicalTrials.gov number, NCT02596126. opens in a new tab; EudraCT Number, 2015-002868-17. opens in a new tab.) |
Comments
A pill containing aspirin and lipid-lowering and blood pressure medications more effectively prevents other adverse cardiovascular events after a heart attack compared to taking the medications separately. That is the finding of a breaking investigation presented in a Hot Line session at the ESC 2022 Congress.
Dr. Valentin Fuster from the National Center for Cardiovascular Research (CNIC), Madrid, Spain and Mount Sinai Health System, New York, USA, said: “The SECURE results show, for the first time, that a polypill containing "Aspirin, atorvastatin, and ramipril lead to clinically relevant reductions in recurrent cardiovascular events in patients who have suffered a myocardial infarction."
After a heart attack, patients are prescribed medications to prevent subsequent cardiovascular events. These include an antiplatelet, a lipid-lowering medication, and a medication to lower blood pressure and stabilize blood vessels. However, less than 50% of post-heart attack patients take all of their medications consistently. It has been proposed that a polypill containing all three treatments would make it easier for patients to comply with their medication.
SECURE was the first randomized trial to study the impact of a polypill on recurrent cardiovascular events in post-infarction patients. The study enrolled patients within six months of suffering a myocardial infarction. Dr. Fuster, principal investigator of the trial, explained: “Most patients become completely adherent after an acute event, but this disappears after the first six months. We wanted to have an impact from the beginning, while all patients were compliant. In fact, most patients in the trial started the polypill in the first week after their myocardial infarction.”
The trial randomly assigned 2,499 patients after a heart attack to a polypill or usual care. The polypill contained aspirin (100 mg), the ACE inhibitor ramipril (2.5, 5, or 10 mg), and atorvastatin (20 or 40 mg). Usual care was at the discretion of the treating physician. The primary composite endpoint was death from cardiovascular causes, nonfatal myocardial infarction, stroke, or urgent revascularization. The Morisky Medication Adherence Scale was used to classify adherence as low, medium, or high.
The average age of the participants was 76 years, 31% were women, 77.9% had hypertension, 57.4% diabetes, and 51.3% had a history of smoking.
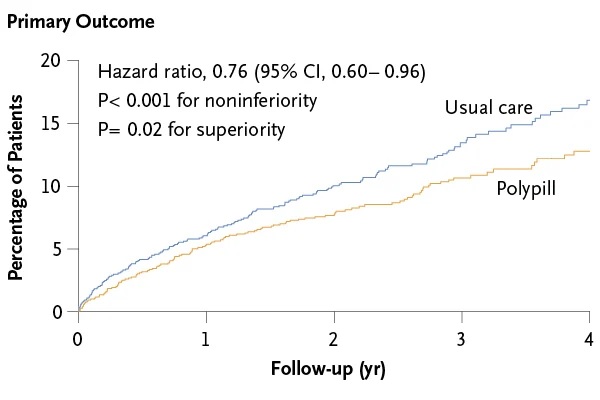
During a median follow-up of three years, the primary composite endpoint occurred in 118 (9.5%) patients in the polypill group and 156 (12.7%) in the usual care group (hazard ratio [HR] 0 .76; 95% confidence interval [CI] 0.60-0.96; p<0.001 for non-inferiority, p=0.02 for superiority).
All four components of the primary endpoint contributed to the observed treatment effect, but the most notable contributor was cardiovascular death, which occurred in 48 (3.9%) patients in the polypill group and 71 (5.8%) in the polypill group. the usual care group (HR 0.67). ;95% CI 0.47-0.97;p=0.03). Treatment effects for the primary outcome were similar in prespecified subgroups (country, age, sex, diabetes, chronic kidney disease,
Regarding secondary endpoints, the key secondary endpoint of cardiovascular death, nonfatal myocardial infarction, or stroke occurred in 101 (8.2%) patients in the polypill group and 144 (11.7%) in the usual care group (HR 0.70; 95% CI 0.54– 0.90; p=0.005). Mortality from all causes was similar in both groups (HR 0.97; 95% CI 0.75-1.25). Patients in the polypill group had higher levels of adherence compared to those in the usual care group.
Dr Fuster said: “The findings suggest that a polypill could become an integral part of strategies to prevent cardiovascular events in patients who have suffered a heart attack. By simplifying treatment and improving adherence, this approach has the potential to reduce the risk of recurrent disease and cardiovascular death on a global scale.”