Key points What is the incidence and significance of cardiac injury in patients with COVID-19? Findings In this cohort study of 416 consecutive patients with confirmed COVID-19, cardiac injury occurred in 19.7% of patients during hospitalization, and was an independent risk factor for in-hospital mortality. Meaning Cardiac injury is a common condition among hospitalized patients with COVID-19, and is associated with an increased risk of in-hospital mortality. |
Summary
Importance
Coronavirus disease 2019 (COVID-19) has resulted in considerable morbidity and mortality worldwide since December 2019. However, information on cardiac injuries in patients affected by COVID-19 is limited.
Aim
Explore the association between cardiac injury and mortality in patients with COVID-19.
Design, environment and participants
This cohort study was conducted from January 20, 2020 to February 10, 2020 at a single center at Renmin Hospital of Wuhan University, Wuhan, China; the final follow-up date was February 15, 2020. All consecutive hospitalized patients with laboratory-confirmed COVID-19 were included in this study.
Main results and measures
Clinical laboratory, radiological, and treatment data were collected and analyzed. The results of patients with and without cardiac injury were compared. The association between cardiac injury and mortality was analyzed.
Heart injury and mortality
Patients with cardiac injury versus those without cardiac injury had a shorter duration from symptom onset to follow-up (mean, 15.6 [range, 1-37] days vs. 16.9 [range, 3-37] ] days; P = .001) and admission to follow-up (6.3 [range, 1-16] days vs. 7.8 [range, 1-23] days; P = .039).
The mortality rate was higher among patients with vs. without cardiac injury (42 [51.2%] vs. 15 [4.5%]; P <.001).
The mortality rate increased in association with the magnitude of the hs-TNI Troponin reference value.
After adjusting for age, pre-existing cardiovascular diseases (hypertension, coronary heart disease and chronic heart failure), cerebrovascular diseases, diabetes mellitus, chronic obstructive pulmonary disease, renal failure, cancer, ARDS, creatinine levels greater than 133 μmol/L and NT - proBNP levels greater than 900 pg/ml, multivariable-adjusted Cox proportional hazard regression model showed a significantly higher risk of death in patients with cardiac injury than in those without cardiac injury, either over time from symptom onset (hazard ratio [HR], 4.26 [95% CI, 1.92-9.49]) or time from entry to study endpoint (HR, 3.41 [95% CI, 1.62-7.16]).
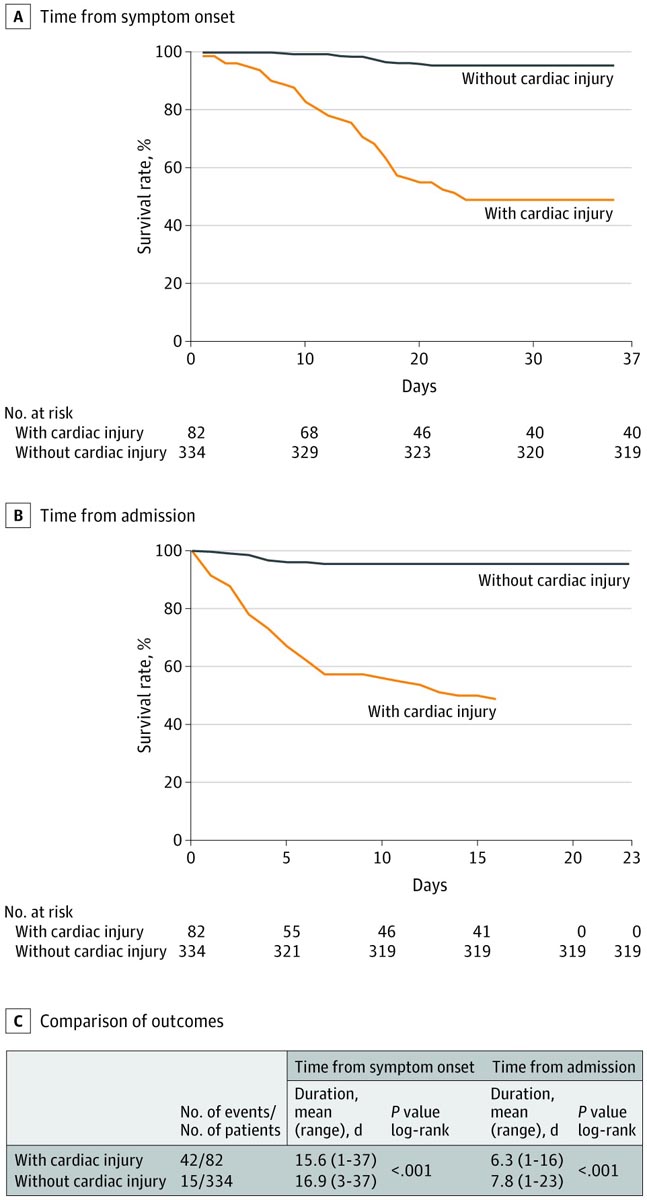
AB, Kaplan-Meier survival curves for mortality during time from symptom onset (A) and admission (B). In (B), the maximum duration was 16 days. C, Patients with cardiac injury had a higher mortality rate in the log-rank test, both from the onset of symptoms and from admission.
Discussion
The present study demonstrates the statistically significant association between cardiac injury and mortality in patients with COVID-19. Cardiac injury, as a common complication (19.7%), was associated with an unexpected high risk of mortality during hospitalization.
As of March 12, 2020, there have been a total of more than 130,000 laboratory-confirmed cases of COVID-19 worldwide, including more than 80,000 in mainland China. Due to its high infectivity, this virus has managed to replace severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) in the death toll.
Severe respiratory distress is generally considered the leading cause of coronavirus-induced death. According to a recent study of the largest clinical sample in China, severe pneumonia was independently associated with admission to an intensive care unit, mechanical ventilation, or death.
Notably, a recent report on 138 patients hospitalized with COVID-19 found that 7.2% of patients developed acute cardiac injury , and patients who received care in the intensive care unit were more likely to have cardiac injury (22.2 %) than those who were not ICU patients. This observation suggests that cardiac injury is possibly associated with clinical outcomes of COVID-19.
Consistently, our study also found that 19.7% of patients with cardiac injury and first demonstrated that cardiac injury was independently associated with an increased risk of mortality in COVID-19 patients. Compared with patients without cardiac injury, patients with cardiac injury had more severe acute illness, manifested by abnormal laboratory and radiographic findings, such as higher levels of C-reactive protein, NT-proBNP, and creatinine levels; more multiple mottling and opacity on ground glass; and a greater proportion requiring non-invasive or invasive ventilation. |
In a study of cardiovascular complications of SARS in 121 patients, hypertension occurred in 61 patients (50.4%) in the hospital. Of these patients, 71.9% developed persistent tachycardia, including 40% with continuous tachycardia during outpatient follow-up. Although tachycardic cardiovascular complications were common in patients with SARS, they were generally self-limited and were not associated with risk of death.
In contrast to that of SARS, more than half of patients with cardiac injury experienced in-hospital death in this study, indicating that COVID-19-induced cardiac injury is associated with important adverse clinical outcomes. However, the mechanism of cardiac injury among these COVID-19 patients remains uncertain.
Evidence from a case report showed that MERS-CoV causes acute myocarditis , which manifests as myocardial edema and acute myocardial injury of the apical and lateral walls of the left ventricle.
This regional myocardial injury may be the result of direct viral infection of the myocardium. Based on recent studies, angiotensin-converting enzyme 2 (ACE2) is a human cellular receptor with strong binding affinity to the Spike protein of SARS-CoV-2, and ACE2 is also highly expressed in the heart. Therefore, it is rational to hypothesize that COVID-19-induced cardiac injury could be mediated by ACE2.
However, a recent pathology study found sparse interstitial mononuclear inflammatory infiltrates in cardiac tissue without substantial myocardial damage in a patient with COVID-19, suggesting that COVID-19 may not directly damage the heart. The present study lacks evidence from MRI or echocardiography to determine the characteristics of myocardial injury. Based on the current results of hs-TNI and ECG findings in a subset of patients, we can only estimate the severity of cardiac injury. Therefore, due to current limited evidence, the question of whether the SARS-CoV-2 virus can directly damage the heart requires further demonstration.
In contrast, a previous study found that reversible subclinical diastolic left ventricular dysfunction appears to be common in acute SARS infection, even among those without underlying heart disease, suggesting that left ventricular dysfunction in the acute phase could be attributable to cytokine storm syndrome .
Cytokine storm This is a serious, life-threatening disease with clinical features of systemic inflammation, methemoglobinemia, hemodynamic instability, and multiple organ failure. The hallmark of cytokine storm syndrome is an uncontrolled and dysfunctional immune response involving the continued activation and proliferation of lymphocytes and macrophages. |
Huang et al found that COVID-19 patients admitted to the intensive care unit had higher plasma levels of cytokines, including interleukin (IL)-2, IL-7, IL-10, granulocyte colony-stimulating factor, IgG-induced protein 10 (also known as CXC motif chemokine 10), monocyte chemoattractant protein 1, macrophage inflammatory protein 1-alpha (also known as chemokine ligand 3), and tumor necrosis factor α.
In the present study, we also found that inflammatory response markers , such as C-reactive protein, procalcitonin, and leukocytes, were significantly increased among patients who sustained cardiac injuries. Enhanced activation or release of these inflammatory cytokines can lead to apoptosis or necrosis of myocardial cells.
Furthermore, pre-existing cardiovascular diseases might also be more susceptible to COVID-19-induced cardiac injury, as approximately 30% and 60% of patients with cardiac injury in the present study had a history of coronary heart disease and hypertension, respectively. , which were significantly more frequent than in those without cardiac injury. Similarly, in a recent report, 25% and 58.3% of patients who were severely ill with COVID-19 had underlying heart disease and hypertension, respectively.
According to the "Diagnosis and Treatment of Novel Coronavirus Pneumonia (Trial Version 4)", elderly patients with underlying diseases are more likely to be infected with SARS-CoV-2 and tend to be severely ill, especially those with hypertension , coronary heart disease and diabetes.
Although there is little evidence to establish a direct association between cardiac injury and cardiovascular comorbidities, it is rational to assume that patients with coronary artery disease or heart failure are susceptible to cardiac injury, and once such patients become infected with pneumonia Severe cardiac ischemia or dysfunction is more likely to occur, ultimately leading to sudden deterioration.
On the other hand, acute inflammatory responses can also lead to ischemia in the presence of pre-existing cardiovascular diseases. Inflammatory activity within coronary atherosclerotic plaques is exacerbated during the systemic inflammatory response, making them prone to rupture .
Inflammation also causes endothelial dysfunction and increases the procoagulant activity of the blood, which may contribute to the formation of an occlusive thrombus over a ruptured coronary plaque. Based on these lines of evidence, we hypothesize that an intense inflammatory response superimposed on pre-existing cardiovascular disease may precipitate the cardiac injury observed in patients with COVID-19 infections.
Conclusions Cardiac injury is a common condition among hospitalized patients with COVID-19, and is associated with an increased risk of in-hospital mortality. Although the exact mechanism of cardiac injury needs to be further explored, the findings presented here highlight the need to consider this complication in the management of COVID-19. |