Summary
Background
The therapeutics have not yet been proven to be effective for the treatment of serious diseases caused by SARS-CoV-2.
Methods
We conducted a randomized, controlled, open-label trial that included hospitalized adult patients with confirmed infection with SARS-CoV-2, which causes the respiratory disease Covid-19, and an oxygen saturation (Sao2) of 94% or less while breathing at room temperature. . air or a ratio of the partial pressure of oxygen (Pao2) to the fraction of inspired oxygen (Fio2) of less than 300 mm Hg.
Patients were randomly assigned in a 1:1 ratio to receive lopinavir-ritonavir (400 mg and 100 mg, respectively) twice daily for 14 days in addition to standard care or standard care alone.
The primary endpoint was time to clinical improvement, defined as the time from randomization to a two-point improvement on a seven-category ordinal scale or discharge from the hospital, whichever came first.
Results
A total of 199 patients with laboratory-confirmed SARS-CoV-2 infection underwent randomization; 99 were assigned to the lopinavir-ritonavir group, and 100 to the standard care group.
Treatment with lopinavir-ritonavir was not associated with a difference from standard care in time to clinical improvement (risk ratio for clinical improvement, 1.24; 95% confidence interval [CI], 0.90 to 1. 72).
Mortality at 28 days was similar in the lopinavir-ritonavir group and the standard care group (19.2% vs. 25.0%; difference, −5.8 percentage points; 95% CI, −17.3 to 5.7) .
The percentages of patients with detectable viral RNA at various time points were similar.
In a modified intention-to-treat analysis, lopinavir-ritonavir led to a shorter median clinical improvement of 1 day than that seen with standard care (hazard ratio, 1.39; 95% CI, 1.00 to 1.91).
Gastrointestinal adverse events were more common in the lopinavir-ritonavir group, but serious adverse events were more common in the standard care group.
Treatment with lopinavir-ritonavir was discontinued early in 13 patients (13.8%) due to adverse events.
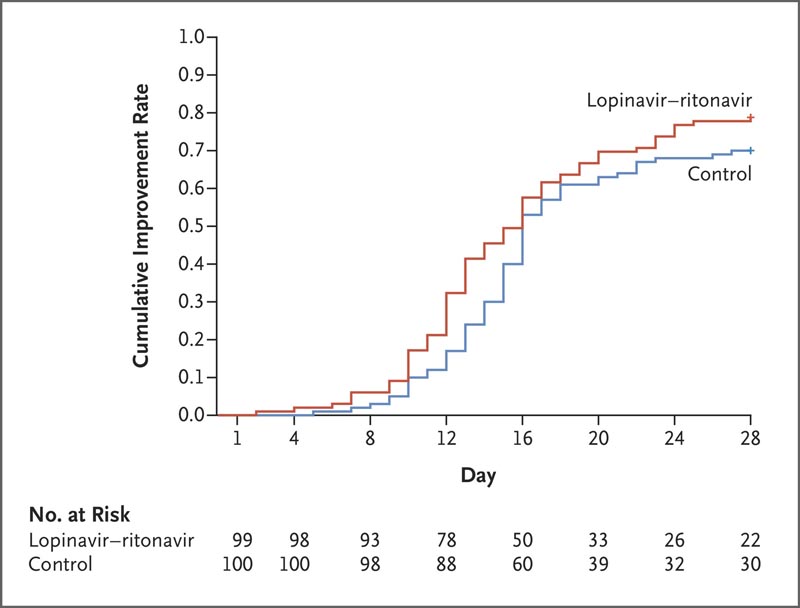
Time to clinical improvement in the intent-to-treat population.
Discussion
This randomized trial found that lopinavir-ritonavir treatment added to standard supportive care was not associated with clinical improvement or mortality in severely ill patients with Covid-19 different from that associated with standard care alone.
However, in the modified intention-to-treat analysis, which excluded three patients with early death, the difference between groups in the median time to clinical improvement (median, 15 days vs. 16 days) was significant, although modest.
Of note, the overall mortality in this trial (22.1%) was substantially higher than the 11% to 14.5% mortality reported in initial descriptive studies of hospitalized patients with Covid-19,1,2, which indicates that we include a seriously ill population . Our patient population was heterogeneous with respect to duration and severity of illness at enrollment;
Accelerated clinical recovery (16.0 days vs. 17.0 days) and reduced mortality (19.0% vs. 27.1%) were observed in a post hoc subgroup of those treated within 12 days of symptom onset, but not in those treated later. The question of whether pretreatment with lopinavir-ritonavir in Covid-19 could have clinical benefit is important and requires further study.
The finding is consistent with studies showing that patients with SARS-CoV-2 viral pneumonia have progression in the second week of illness and with time-to-treatment effects observed in previous antiviral studies in SARS-COV2 and severe influenza. . Furthermore, we found that the number of lopinavir-ritonavir recipients who had serious complications (acute kidney injury and secondary infections) or who required invasive or non-invasive mechanical ventilation for respiratory failure was lower than in those who did not receive treatment.
These observations are hypothesis-generating and require additional studies to determine whether treatment with lopinavir-ritonavir administered at a certain stage of the disease can reduce some complications in Covid-19.
We did not find that adding lopinavir-ritonavir treatment reduced viral RNA loads or the duration of viral RNA detectability compared with standard supportive care alone. SARS-CoV-2 RNA was still detected in 40.7% of patients in the lopinavir-ritonavir group at the end of the trial (day 28). A recent report showed that the median duration of virus shedding in Covid-19 was 20 days in patients with severe illness and could last up to 37 days.
Neither that study nor the current one found evidence that lopinavir-ritonavir exerted a significant antiviral effect. The reasons for the apparent lack of antiviral effect are uncertain, but the sampling methods used in the current trial were probably not optimal. Samples were taken only intermittently (days 1, 5, 10, 14, 21, and 28), and more frequent sampling in the first 5 days could have provided a more detailed characterization of the viral load kinetics in the two groups during this critical period.
Furthermore, previous studies have shown that throat swab samples have lower viral loads than nasopharyngeal samples, and more importantly, we were unable to sample lower respiratory tract secretions.
Of note, depending on the cell type used, 50% effective concentrations (EC50) of lopinavir in vitro for SARS-CoV have ranged between 4.0 and 10.7 μg per milliliter, 5,6,8, although other studies have reported that lopinavir was inactive26 or higher concentrations (25 μg per milliliter) were required for inhibition.
For MERS-CoV, EC50 values ranged from 5 to approximately 7 μg per milliliter. Both the mean peak (9.6 μg per milliliter) and trough serum concentrations (5.5 μg per milliliter) of lopinavir in adults alone approach these concentrations. Whether the EC50 value is an appropriate threshold and whether unbound lopinavir concentrations in human plasma are sufficient for SARS-CoV-2 inhibition are questionable.
Nearly 14% of lopinavir-ritonavir recipients were unable to complete the full 14-day course of administration. This was mainly due to gastrointestinal adverse events , including anorexia, nausea, abdominal discomfort or diarrhea, as well as two serious adverse events, both acute gastritis. Two recipients had self-limited rashes.
Such side effects, including the risks of liver injury, pancreatitis, more severe skin rashes, and QT prolongation, and the potential for multiple drug interactions due to CYP3A inhibition, are well documented with this drug combination. The side effect profile observed in the current trial raises concerns about the use of higher or longer lopinavir-ritonavir dosing regimens in an effort to improve outcomes.
Conclusions
|
(Funded by the National Major Science and Technology Projects on Creation and Development of New Drugs and others; Chinese Clinical Trial Registration number, ChiCTR2000029308. Opens in a new tab.)
















