Summary Background Polyclonal convalescent plasma can be obtained from donors who have recovered from coronavirus disease 2019 (Covid-19). The effectiveness of this plasma in preventing serious complications in outpatients with recent-onset Covid-19 is uncertain. Methods In this multicenter, double-blind, randomized, controlled trial, we evaluated the efficacy and safety of Covid-19 convalescent plasma, compared to control plasma, in symptomatic adults (≥18 years of age) who tested positive for the severe acute respiratory disease coronavirus 2 syndrome, regardless of their risk factors for disease progression or vaccination status. Participants were enrolled within 8 days of symptom onset and received a transfusion within 1 day after randomization. The primary outcome was Covid-19-related hospitalization within 28 days of transfusion. Results Participants were enrolled from June 3, 2020 to October 1, 2021. A total of 1,225 participants were randomized and 1,181 received a transfusion. In the prespecified modified intention-to-treat analysis that included only participants who received a transfusion, the primary outcome occurred in 17 of 592 participants (2.9%) who received convalescent plasma and 37 of 589 participants (6.3%) who received control plasma (absolute risk reduction, 3.4 percentage points; 95% confidence interval, 1.0 to 5.8; P = 0.005), corresponding to a relative risk reduction of 54%. No evidence of efficacy in vaccinated participants can be inferred from these data because 53 of the 54 participants with Covid-19 who were hospitalized were unvaccinated and 1 participant was partially vaccinated. A total of 16 grade 3 or 4 adverse events (7 in the convalescent plasma group and 9 in the control plasma group) occurred in participants who were not hospitalized. Conclusions In participants with Covid-19, most of whom were unvaccinated, administering convalescent plasma within 9 days of symptom onset reduced the risk of disease progression leading to hospitalization. |
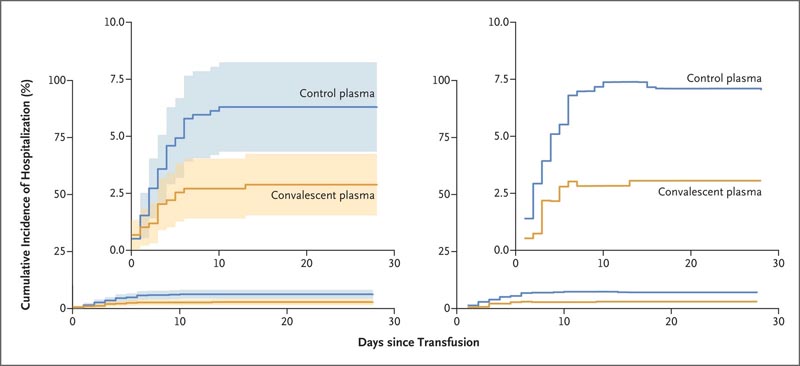
Cumulative incidence of hospitalization related to coronavirus disease 2019. Results from the unadjusted analysis are shown on the left. Shading indicates the 95% confidence interval. On the right are the estimates according to the estimation model based on adjusted minimum focused losses. The boxes show the same data on an expanded y-axis.
Comments
Peer-reviewed publication in the New England Journal of Medicine validates findings first announced in December
The New England Journal of Medicine (NEJM) today published the final results of a nationwide multicenter study led by researchers at Johns Hopkins Medicine and the Johns Hopkins Bloomberg School of Public Health showing plasma from patients who have recovered from COVID-19 and whose blood contains antibodies against SARS-CoV-2, the causative virus, is an effective and safe option as an early outpatient treatment for the disease.
Research showed that high-titer (antibody-rich) COVID convalescent plasma, when administered to COVID-19 outpatients within nine days of testing positive, reduced the need for hospitalization for more than half of the predominantly unvaccinated outpatients of the study.
The U.S. Food and Drug Administration (FDA) currently authorizes this plasma as a treatment option for outpatients with immunocompromising conditions or receiving immunocompromising medications, and for all hospitalized patients with early-stage COVID-19.
The findings were first presented in a preprint posted on MedRxiv on December 21, 2021. Details of the study, including authors and funding sources, can be found in the Johns Hopkins press release issued at that time.
“Based on our findings and conclusions, which are now validated through the peer review process, we encourage healthcare professionals to keep SARS-CoV-2 antibody-rich blood plasma available in their blood banks.” as part of the treatment arsenal against early disease. COVID-19 stage,” says study co-senior author David Sullivan, MD, professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health with a joint appointment in infectious diseases at the Johns Hopkins University School of Medicine. .
“We believe the best role for convalescent plasma is to extend its use to early outpatient treatment when other therapies, such as monoclonal antibodies or medications, are not readily available, such as in low- and middle-income countries, or are ineffective, such as "It occurs with variants of SARS-CoV-2 that are resistant to certain monoclonal antibodies," adds Sullivan.
In the outpatient early treatment study conducted between June 2020 and October 2021, researchers gave 1,181 randomly assigned patients a dose of high-titer polyclonal convalescent plasma (containing a concentrated mixture of SARS-specific antibodies). CoV-2) or placebo control plasma (without antibodies against SARS-CoV-2). The patients were 18 years or older and had tested positive for SARS-CoV-2 within eight days before the transfusion. A successful therapy was defined as a patient who did not require hospitalization within 28 days after plasma transfusion.
The study found that 17 patients of 592 (2.9%) who received convalescent plasma required hospitalization within 28 days of transfusion, compared with 37 of 589 (6.3%) who received placebo control plasma. This resulted in a relative risk reduction of hospitalization of 54%.
The timing of convalescent plasma transfusion is also critical: "The sooner the better," the researchers say.
“Based on findings from an analysis in the new paper that was not available when the preprint was published, we found that if convalescent plasma is given within five days of diagnosis, the effectiveness in reducing hospitalization approaches 80%.” says Sullivan.
"We conclude that these results strongly support high-titer SARS-CoV-2 convalescent plasma as an effective early treatment for COVID-19 with advantages such as low cost, wide availability, and rapid resistance to evolving variants of the virus," says the Study co-senior author Kelly Gebo, MD, MPH, professor of medicine at Johns Hopkins University School of Medicine.
The next step, researchers say, is to make convalescent plasma for outpatient COVID-19 treatment easier to use, administered more efficiently and more accessible to those who need it. As part of that effort, they have provided physicians with guidance for implementing a COVID-19 outpatient plasma transfusion center, including logistical, staffing, and blood banking requirements. The guide appears in an article published on March 29, 2022 in the magazine Transfusion.
The team also continues to seek a greater understanding of what else convalescent plasma can do for outpatients with COVID-19. A soon-to-be published study will look at the ability of plasma to neutralize SARS-CoV-2 variants, including delta and omicron, despite no prior donor exposure to those viruses.
Since the study findings were first announced last December, there have been three developments supporting the use of convalescent plasma for early stage COVID-19:
- On December 28, 2021, the FDA expanded the authorized emergency use of convalescent plasma with high titers of anti-SARS-CoV-2 antibodies “for the treatment of COVID-19 in patients with immunosuppressive disease or receiving immunosuppressive treatment, as whether in the outpatient or inpatient setting.
- On February 2, 2022, the Infectious Diseases Society of America updated its "Guidelines for the Treatment and Management of Patients with COVID-19" to include the "use of convalescent plasma in outpatients with mild to moderate COVID-19 in high risk of progression to severe disease without other treatment options.”
- On March 7, 2022, the American Red Cross announced that it was “temporarily testing all blood donations for COVID-19 antibodies to help identify donations that could be processed into convalescent plasma.” The organization said this was being done “to help support immunocompromised patients fighting COVID-19.”
“These recent recognitions of the benefit of high-titer convalescent plasma in treating early-stage COVID-19, along with our peer-reviewed findings and our new guidance for more effective treatment delivery, provide clinicians with a additional option for outpatients,” says Gebo.