Purpose of the review
Coronavirus disease 2019 (COVID-19) has been the cause of significant morbidity and mortality worldwide. Here, we review the literature to date on the short- and long-term consequences of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) heart infection.
Recent findings
Early case reports described a spectrum of cardiovascular manifestations of COVID-19, including myocarditis, stress cardiomyopathy, myocardial infarction, and arrhythmia. However, in most cases, myocardial injury in COVID-19 appears to be predominantly mediated by the severity of critical illness rather than direct myocardial injury by viral particles.
While cardiac MRI remains a powerful tool for diagnosing acute myocarditis, it should be used cautiously in light of the low baseline prevalence of myocarditis.
Guiding a patient athlete to return to sport (RTP) after COVID-19 infection is a challenging process. The most recent data shows that RTP has been a secure endeavor using a detection protocol.
“Long COVID” or post-acute sequelae of SARS-CoV-2 infection have also been described . Reported symptoms encompass a wide range of cardiopulmonary and neurological complaints including fatigue, palpitations, chest pain, dyspnea, mental confusion, and dysautonomia, including postural tachycardia syndrome (POTS). Management of POTS/dysautonomia focuses primarily on education, exercise, and salt and fluid replacement.
Summary
Our understanding of the impact of COVID-19 on the cardiovascular system is constantly evolving. As we enter a new era of survival, additional research is needed to catalog the burden of persistent cardiopulmonary symptoms. Research is also needed to understand how acute management may alter the likelihood and prevalence of this chronic syndrome.
In 2020, coronavirus disease 2019 (COVID-19) was the third leading cause of death with an estimated 345,323 deaths in the U.S. Perhaps more than any other communicable disease, COVID-19 has captivated the community of cardiology due to its apparent links to cardiovascular diseases (CVD).
The novelty of the virus led to an early reliance on small case reports and theoretical explanations to explain and predict the impact on CVD. Now, more than a year since the start of the pandemic, more mature studies have emerged that refine our understanding of the interaction between COVID-19 and the heart.
At the beginning of the pandemic, patients with cardiovascular comorbidities were more vulnerable to serious infections. The specificity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for the angiotensin-converting enzyme-2 (ACE-2) protein fueled further concerns about injury to the cardiovascular system and raised fears about concurrent use of drugs, including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Early case reports described a spectrum of cardiovascular manifestations of COVID-19 infection, including myocarditis, stress cardiomyopathy, myocardial infarction (MI), and arrhythmia. To combat a new disease, the cardiology community deployed its most advanced technology, including cardiac magnetic resonance (CMR), which has characterized the acute and chronic consequences of SARS-CoV-2 infection, but the findings have often left to doctors with more questions than answers.
Now, more than a year since the first reported cases in 2020, the global community finds itself at a critical point in the pandemic timeline. With survivors outnumbering the infected and vaccines in distribution, more attention may be paid to the long-term cardiovascular effects of COVID-19.
However, as surges continue around the world due to new variants and a delay in vaccine distribution, the medical community must remain informed about the latest evidence-based management of acute COVID-19 infection.
Part I: Acute infection
Mechanisms of cardiac injury in COVID-19
Cardiac troponin is a highly specific test for myocardial injury, which can be measured by conventional or highly sensitive assays. In particular, an elevated troponin (defined as being above the 99th percentile of the upper reference limit) does not necessarily equate to an MI. According to the 4th universal definition, the criteria for an MI require a troponin rise/fall pattern with at least one value above the 99th percentile along with other symptoms or signs of ischemia.
| A type 1 myocardial infarction (MI) occurs from an acute plaque rupture/erosion event, which has also been seen in the context of other viral infections, while a type 2 MI is from "demand ischemia" in the context of an oxygen demand/supply mismatch arising from stressors such as hypoxia, hypoperfusion and tachycardia, which can occur in COVID-19, as well as other critical illnesses. Both types of MI have been reported in COVID-19. |
However, paradoxically, there was an approximate 20% reduction in ST-elevation myocardial infarction (STEMI) rates during the COVID-19 pandemic. Alternative mechanisms have been postulated behind this reduction in STEMI, but the main concern was that patients were avoiding hospital care for fear of contracting the virus.
Beyond acute myocardial infarction, troponin elevation may accompany a number of other cardiovascular presentations of COVID-19 including viral myocarditis, indirect cardiac injury from cytokine storm, stress cardiomyopathy, heart failure (HF), stroke pulmonary disorders and arrhythmias, or reflect CVD or cardiac structural abnormalities.
The prevalence of cardiac injury, as measured by elevated cardiac troponin, on the order of 20-40% among the first reported patients with severe COVID-19 (hospitalized) attracted the attention of cardiology and the broader medical community. As the virology of SARS-CoV-2 became clear, its interaction with the ACE2 protein found in cardiomyocytes supported the physiological plausibility of direct cardiac viral injury .
A precedent had been established with a related coronavirus, SARS-CoV-1, which caused the first SARS outbreak in Asia, whereby viral RNA was isolated in heart tissue. Additionally, individuals with CVD, such as coronary artery disease (CHD) and HF, and those with CVD risk factors such as hypertension, diabetes, and obesity were shown to be more susceptible to serious infections, raising concerns that the heart may be a direct viral target and become more vulnerable if compromised.
Regarding the etiology of myocardial injury in COVID-19, our knowledge has evolved since the beginning of the outbreak. Larger histopathological studies have challenged early frameworks of cardiac injury, demonstrating that the prevalence of myocarditis and direct viral toxicity to myocytes are extremely rare .
In one of the largest cardiac autopsy series to date, Lindner et al. They showed that, although viral RNA was isolated in cardiac tissue, in situ hybridization localized the site of infection not to cardiomyocytes, but to the interstitium and infiltrating macrophages. Additionally, there were zero confirmed cases of myocarditis according to the Dallas criteria. Other pathological studies have also failed to document direct infection of cardiomyocytes.
In particular, as the characteristics of the new coronavirus were quickly cataloged at the beginning of the pandemic, little was done to compare them with appropriate control groups. Recent research has placed COVID-19 in the context of the broader critical care landscape.
Metkus and colleagues compared troponin elevation in COVID-19 acute respiratory distress syndrome (ARDS) versus non-COVID-19 ARDS among nearly 250 intubated patients in a large hospital system and showed that myocardial injury was actually less common in COVID-19 than in non-COVID-19 ARDS patients after taking into account the degree of critical illness and organ dysfunction. COVID-19 patients had worse oxygenation and hemodynamics, reinforcing indirect cardiac injury secondary to critical illness as the most likely mechanism at play.
These findings are reinforced by the high rates of myocardial injury seen in systemic infections other than COVID-19, including sepsis, documented in the critical care literature.]
While other cardiac manifestations such as myocarditis, stress cardiomyopathy, and myocardial infarction have been described in COVID-19 and should not be discounted, placing COVID-19 in the context of other critical illnesses has recalibrated our understanding of the injury. myocardial to recognize more prevalent mechanisms such as hypoxemia and hemodynamic compromise.
Although myocardial injury in COVID-19 may not be unique to the virus, the degree of critical illness it can cause speaks to unique pathogenic attributes.
The mechanism responsible is likely related to its ability to stimulate a robust inflammatory response . In studies of myocardial injury in COVID-19, predictors of troponin elevation consistently demonstrated associations with inflammatory markers, including C-reactive protein (CRP), D-dimer, ferritin, and fibrinogen. Pathology studies have supported this relationship by demonstrating increased cytokine expression with higher viral loads.
While the hyperinflammatory phase inflicts much of the respiratory and circulatory compromise that mediates indirect myocardial injury in severe infection, inflammation was previously known to directly mediate CVD, as seen in atherosclerosis and other hyperinflammatory states, including sepsis and hemophagocytic lymphohistiocytosis (HLH).
Cardiomyocytes express cytokine receptors, including tumor necrosis factor and interleukin-6, the effects of which can reduce inotropy secondary to alterations in catecholamine signaling and cause cytotoxic damage. Furthermore, cytokines alter vascular endothelium to promote inflammatory migration and may cause endothelitis, microthrombi, and microvascular injury that have been described in COVID-19.
Echocardiography has further refined our understanding of myocardial damage in COVID-19, detailing certain functional patterns of injury . Szekely et al. found that right ventricular (RV) dysfunction was the most common echocardiographic abnormality in a series of 100 hospitalized patients with COVID-19, among almost 40%, with RV deterioration most associated with clinical decompensations. RV dysfunction was also the most common abnormality observed in a multicenter international cohort of more than 300 hospitalized patients with COVID-19, about 26%.
However, in both studies, a full spectrum of dysfunction was observed, including global and regional left ventricular (LV) systolic dysfunction, diastolic dysfunction, and pericardial effusions. The prevalence of RV dysfunction indicates that COVID-19 is a predominantly respiratory pathogen with a propensity for deep vein thrombosis and pulmonary embolism, all of which can compromise pulmonary vascular resistance and increase RV loading conditions.
Troponin elevation: prognostic implications
Leaving aside the mechanism of injury, detectable troponin elevation has prognostic value in acute COVID-19 infection. Shi and his colleagues were among the first to report increased mortality in those with elevated troponin from a single-center cohort in Wuhan, finding a three- to fourfold risk of death.
Subsequently, Lombardi et al. They validated these findings in a multicenter cohort in Italy with more than 600 patients, although with a more attenuated hazard ratio of 1.7. In one of the most diverse cohorts studied with more than 2000 patients admitted to a New York City hospital system, Smilowitz et al. They illustrated that the risk of death was twice as high among patients with troponin elevation.
Importantly, the degree of troponin elevation was associated with more severe critical illness (defined as ICU admission, need for mechanical ventilation, or death).
While these seminal studies defined troponin elevation as greater than the 99th percentile of the upper limit of normal, Qin and colleagues illustrated that troponin elevation in COVID-19 infection was associated with mortality even at low thresholds. 19 to 50% lower than those traditionally used in settings.
Furthermore, the risk of mortality and adverse outcomes appears to be continuous with the degree of troponin elevation; Higher troponin continues to amplify risk, providing clinicians with a quantitative and not just qualitative risk assessment for patients. As such, troponin measurement for hospitalized patients with COVID-19 has been integrated into routine clinical practice and management algorithms.
In the case of hospitals, it serves to forecast the trajectory and identify patients who may require more intensive resources, especially in times of shortage. Several society guidelines, including the World Health Organization and the Chinese Clinical Guideline for COVID-19, recommend measuring troponin for all admitted patients, while others, including the American College of Cardiology (ACC), recommend testing when clinically indicated.
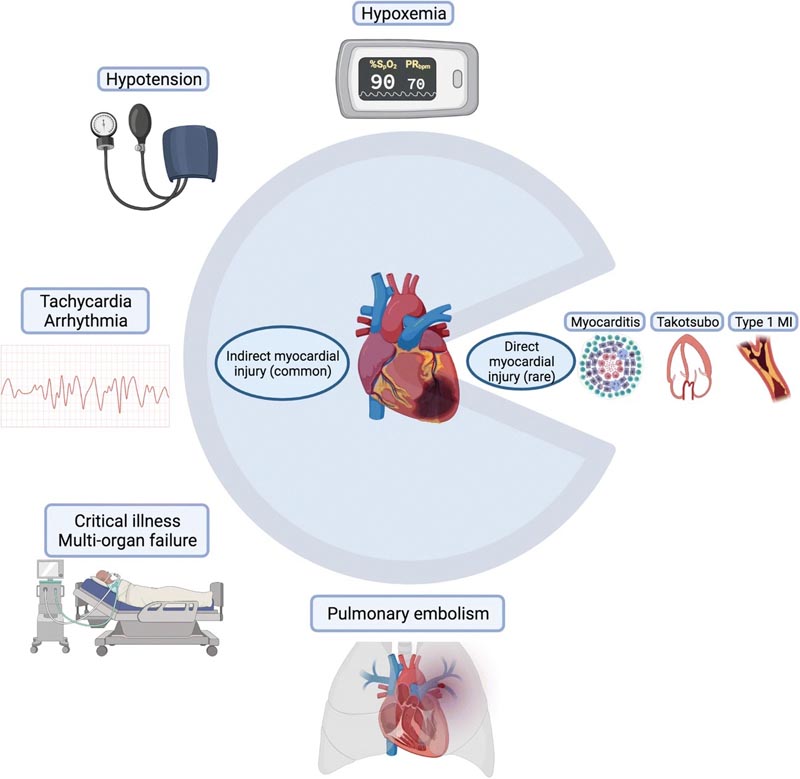
Symbolic pie chart illustrating common causes of indirect myocardial injury and rarer causes of direct myocardial injury in COVID-19 infection
Role of cardiac magnetic resonance (CMR)
The pathophysiological plausibility of COVID-19 causing direct myocardial infection and early case reports invoking myocarditis led to increased interest in the use of CMR, now the preferred non-invasive diagnostic modality for acute myocarditis. Early case reports of myocarditis in acute COVID-19 infection reported a prevalence of around 7%; however, these studies were flawed by inconsistent diagnostic criteria and limited sample sizes.
As discussed above in the context of histopathological studies, the prevalence of myocarditis in COVID-19 is now considered extremely rare , and larger retrospective multicenter cohort studies have found it to be 1% or less.
Recognizing the low pretest probability of COVID-19 myocarditis and considering the most likely causes of myocardial injury inform the appropriate use of CMR. Particularly with CMR, the longer scan time and logistics required to accommodate examinations in intubated patients (including patients transitioning to and from scanner-safe ventilators) increase the risk of exposure for healthcare workers.
Part II: Resolved COVID-19 Infection
and Return to Play for Athletes
The question of when a competitive athlete can return to play (RTP) after COVID-19 infection poses an urgent and important challenge for the field of cardiology. The urgency is driven by the fact that sports organizations, from professional to recreational, were some of the first to return at full speed during the pandemic. This collective rush to return began with little data on how to do so safely after an infection.
The importance was clear, as myocarditis is a possible sequelae of COVID-19 infection and a cause of death in young athletes. Exercising with active myocarditis can lead to increased inflammation and a proarrhythmogenic environment . Additionally, the athletic heart may have abnormalities in size, function, and response to exercise that make it difficult to distinguish it from the inflamed or injured heart.
Intense exercise may cause transient elevations in troponins and imaging findings suggesting cardiac fatigue and myocardial inflammation. With thousands of athletes eager to return to action, how to do so safely became a central topic in the field of cardiology throughout the pandemic.
In May 2020, the ACC Section of Sports and Exercise Cardiology issued its first set of RTP recommendations. For those athletes who experienced a symptomatic infection, they recommended a 2-week rest period after resolution of symptoms, cardiac evaluation (electrocardiogram, echocardiogram, or high-sensitivity troponin), and additional cardiac imaging with any abnormalities.
If myocarditis was detected, physicians were referred to the existing American Heart Association (AHA)/ACC myocarditis guidelines, which recommend abstaining from sports for 3-6 months . Six months later, the Section updated and expanded these guidelines to include specific age-based recommendations and detailed troponin and CMR screening recommendations. An Expert Consensus Statement followed, recommending against the use of CMR-based screening of all athletes with prior COVID-19 infection.
Fortunately, the most recent registration data shows that the national RTP has been a safe endeavor. A study of 789 professional athletes who underwent an RTP cardiac testing protocol after COVID-19 infection found imaging evidence of inflammatory heart disease in 5 athletes (0.6%). The cardiac screening protocol included troponin, ECG, and transthoracic echocardiogram; CMR or stress echocardiography was performed only in athletes with an abnormal initial cardiac screening .
No adverse cardiac events occurred in athletes who underwent cardiac testing and returned to play.
Guiding a patient through return to athletics after COVID-19 infection is a challenging process. COVID-19 data is evolving rapidly, sports cardiology is a relatively nascent field, and the athletic heart is a unique substrate. This combines to produce more uncertainty than clear answers when approaching return to play. However, as time has passed and more data has emerged, that return, when guided by current screening recommendations, can be done safely.
long COVID
As healthcare workers and researchers continue to learn, classify and treat the acute cardiovascular risks of COVID-19, many outpatient providers are being inundated by patients with persistent symptoms after an acute infection, known in popular media as “ “Long COVID . ”
With greater recognition of this syndrome ongoing, researchers have established the following definitions:
- Post-acute COVID syndrome (PACS) for persistent symptoms after 3 weeks.
- Chronic COVID syndrome: after 12 weeks.
The National Institutes of Health has also referred to “long COVID” as post-acute sequelae of SARS-CoV-2 infection (PASC). Reported symptoms encompass a wide range of cardiopulmonary and neurological complaints, including fatigue, palpitations, chest pain, dyspnea, mental confusion, and dysautonomia.
While early studies estimated the prevalence of long COVID at between 30 and 80%, they were limited by a primary focus on hospitalized patients. Within a non-hospitalized cohort of 272 people in the US, 35% reported not being at baseline 14-21 days after COVID-19 diagnosis.
New studies are using mobile technology to allow responders to directly monitor and report their symptoms for both acute and long-term symptom tracking. While older people with multiple comorbidities are at higher risk for long COVID, approximately 20% of younger people, ages 18 to 34 and without comorbid conditions, also continued to report ongoing symptoms at 14-21 days.
Regarding specific cardiovascular symptoms, approximately 20% of individuals reported chest pain and 14% reported palpitations at 60 days. Inflammation and increased metabolic and myocardial demand are thought to contribute to the persistence of cardiovascular symptoms, as has been observed in other severe coronavirus infections such as SARS.
A growing number of patients and case studies are also noting a relationship between COVID-19 and postural orthostatic tachycardia syndrome (POTS). POTS is characterized by changes in heart rate with changes in position, often accompanied by palpitations and decreased exercise tolerance. POTS has previously been linked to post-viral diseases, but the exact mechanism is unknown.
One hypothesis connecting POTS to COVID-19 is based on its known interaction with the ACE2 protein expressed in neurons. The investigators hypothesize that this may disrupt normal ACE2-mediated blood pressure regulation, leading to hypotension and dysautonomia. Management of POTS and dysautonomia focuses primarily on education, exercise, and salt and fluid replacement. Agents such as midodrine can improve vascular tone, while beta blockers and ivabradine can help control palpitations.
With 20-30% of outpatients and up to 80% of hospitalized patients with persistent symptoms, providers and researchers now have the responsibility to recognize and manage the persistent burden of COVID-19 infection. Many recover slowly on their own through anticipatory guidance and light exercise.
However, the British Thoracic Society has established guidelines for following up all patients , regardless of severity, at 12 weeks with a chest x-ray and clinical assessment to assess the need for further testing.
People with severe COVID-19 are recommended to follow up earlier, at 4-6 weeks, to assess the need for further testing and multidisciplinary rehabilitation. Serial electrocardiograms and echocardiograms can be used to monitor people with persistent cardiac symptoms, although advanced imaging should be analyzed on a case-by-case basis.
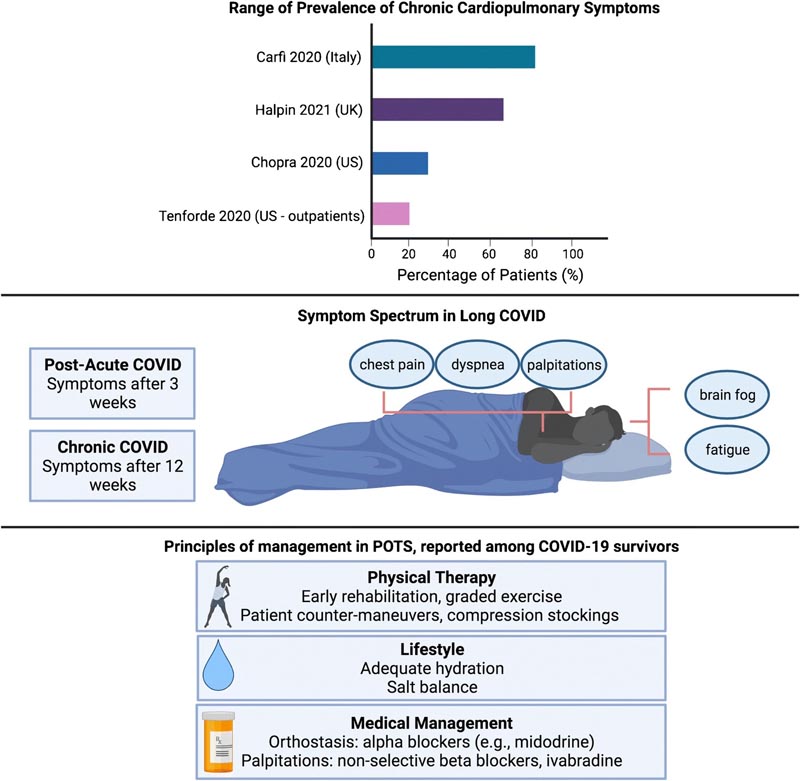
Review of the prevalence of long COVID, definitions and symptomatic manifestations, and current treatment principles for possible overlap of COVID/postural orthostatic tachycardia syndrome
Conclusion It has been some time since the global medical community has faced a new disease of pandemic proportions. Unraveling the mysteries of COVID-19 has been an exercise in diligent science and experimentation. The latest observational, pathological, imaging and clinical studies have clarified the short- and long-term impacts of COVID-19 on the cardiovascular system and updated our understanding in several ways. Myocardial injury in COVID-19 appears to be predominantly mediated by the severity of critical illness rather than direct myocardial injury by viral particles. While myocardial injury is not unique to COVID-19 and is seen elsewhere in the critical care literature in sepsis and ARDS, the hyperinflammatory response precipitated by COVID-19 is a unique hallmark and may mediate the more severe clinical courses. observed in comparison with other viruses. If anything, COVID-19 has reinforced the critical interplay between inflammation and CVD and should drive future work in this field. While myocardial injury in the form of troponin elevation is prevalent and prognostic in acute COVID-19 infection, recent studies suggest that troponin elevation is a marker of disease severity and underlying substrate, rather than a mediator. independent of the results. While CMR remains a powerful tool for diagnosing acute myocarditis, it should be used cautiously in light of the low baseline prevalence established in studies to date, as well as the risk of exposure to healthcare personnel. Studies are needed to understand the clinical relevance of the persistent inflammatory signals seen in survivors and how this may be compared or contrasted with those recovering from other common viruses or critical illnesses. The RMC may have a more focused role in providing recommendations for at-risk populations such as athletes; however, it should rarely be the first-line modality and imaging findings alone should not serve as a basis for the diagnosis of acute myocarditis. Finally, as we enter a new era of survivorship, additional research is needed to catalog the burden of persistent cardiopulmonary symptoms that have significant implications for patient well-being and global economies regarding the ability to return to work. Research is also needed to confirm whether existing therapies for dysautonomia, including POTS, are effective in the long COVID population, and how acute management may alter the likelihood and prevalence of this chronic syndrome. While questions remain and will continue to arise regarding COVID-19 and CV diseases, the pandemic has shown that the scientific community is uniquely engaged and capable of providing these critical answers. |