Background
The cardiovascular safety of testosterone replacement therapy in middle-aged and older men with hypogonadism has not been determined.
Methods
In a multicenter, randomized, double-blind, placebo-controlled, noninferiority trial, we enrolled 5246 men aged 45 to 80 years who were at high or preexisting risk for cardiovascular disease and who reported symptoms of hypogonadism and had two levels of Fasting testosterone of less than 300 ng per deciliter.
Patients were randomly assigned to receive daily 1.62% transdermal testosterone gel (dose adjusted to maintain testosterone levels between 350 and 750 ng per deciliter) or placebo gel.
The primary cardiovascular safety end point was the first occurrence of any component of a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, assessed in a time-to-event analysis.
A secondary cardiovascular endpoint was the first occurrence of any component of the composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization, assessed in a time-to-event analysis. Noninferiority required an upper limit of less than 1.5 for the 95% confidence interval of the hazard ratio among patients who received at least one dose of testosterone or placebo.
Results
The mean (±SD) duration of treatment was 21.7±14.1 months and the mean follow-up was 33.0±12.1 months. A primary cardiovascular endpoint event occurred in 182 patients (7.0%) in the testosterone group and 190 patients (7.3%) in the placebo group (hazard ratio, 0.96; range 95% confidence, 0.78 to 1.17; P<0.001 for noninferiority).
Similar findings were observed in sensitivity analyzes in which data on events at various time points after stopping testosterone or placebo were censored.
The incidence of secondary endpoint events or each of the composite primary cardiovascular endpoint events appeared to be similar in the two groups . A higher incidence of atrial fibrillation, acute kidney injury, and pulmonary embolism was observed in the testosterone group.
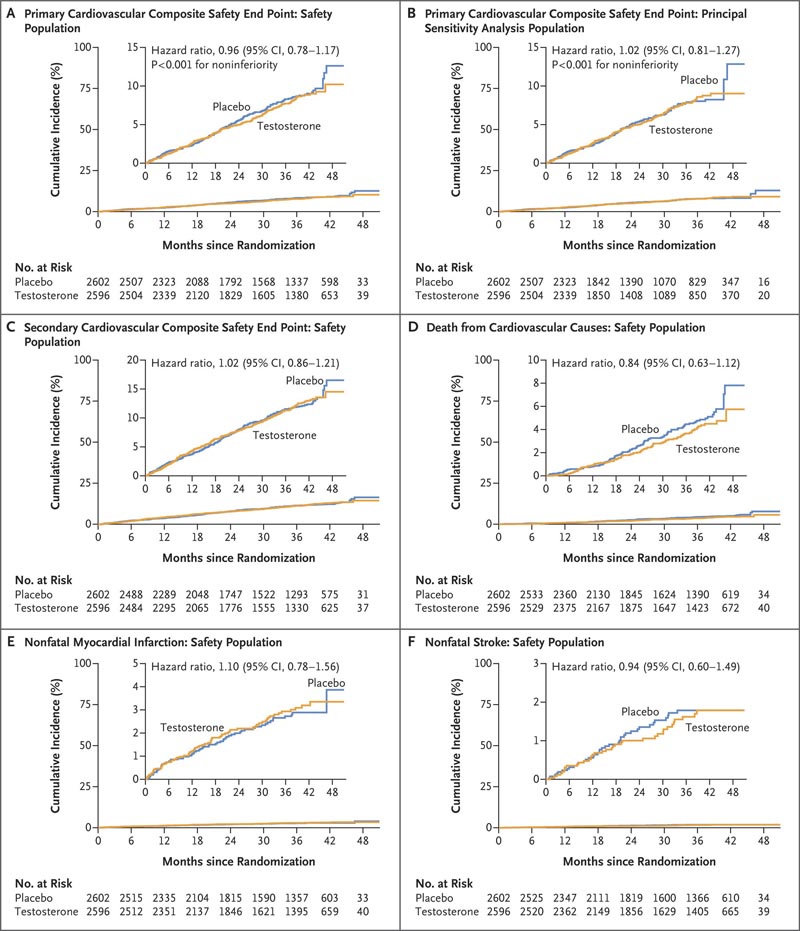
Figure : Time-to-event analysis for primary and secondary cardiovascular safety endpoints . Panel A shows the cumulative incidence of the primary composite cardiovascular safety endpoint, which was defined as death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Panel B shows the cumulative incidence of the primary composite cardiovascular safety endpoint in the primary sensitivity analysis population in which data on endpoint events that occurred more than 365 days after discontinuation were censored. testosterone or placebo. Panel C shows the cumulative incidence of the secondary composite cardiovascular safety endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization; Panel D, the cumulative incidence of death from cardiovascular causes; Panel E, the cumulative incidence of nonfatal myocardial infarction; and Panel F, the cumulative incidence of nonfatal stroke. In each case, the cumulative incidence was estimated with the Kaplan-Meier method and the hazard ratio was calculated with the Cox proportional hazards regression model with adjustment for preexisting cardiovascular disease (yes or no) as a covariate. Because the statistical analysis plan did not include a provision to correct for multiplicity when testing for secondary or other endpoints, results are reported as point estimates and 95% confidence intervals. The widths of the confidence intervals have not been adjusted for multiplicity, so the intervals should not be used to infer definitive treatment effects for secondary endpoints. The insets show the same data on a zoomed-in y-axis.
Conclusions In men with hypogonadism and preexisting cardiovascular disease or at high risk for cardiovascular disease, testosterone replacement therapy was noninferior to placebo with respect to the incidence of major adverse cardiac events. |
(Funded by AbbVie et al.; TRAVERSE ClinicalTrials.gov number, NCT03518034. opens in new tab.)
















