Cardiovascular complications are rapidly emerging as a key threat in coronavirus disease 2019 (COVID-19) in addition to respiratory disease. However, the mechanisms underlying the disproportionate effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with cardiovascular comorbidities remain incompletely understood. 2
SARS-CoV-2 infects the host using the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed in several organs, including the lungs, heart, kidneys, and intestine. ACE2 receptors are also expressed by endothelial cells.
It is currently unknown whether the vascular disorders in COVID-19 are due to the involvement of endothelial cells by the virus. Interestingly, SARS-CoV-2 can directly infect human blood vessel organoids engineered in vitro.4
Here we demonstrate the involvement of endothelial cells across vascular beds of different organs in a series of COVID-19 patients.
Patient 1 was a 71-year-old male kidney transplant recipient with coronary artery disease and hypertension. The patient’s condition deteriorated after the COVID-19 diagnosis, and he required mechanical ventilation. Multisystem organ failure occurred and the patient died on day 8.
Post-mortem analysis of the transplanted kidney by electron microscopy revealed viral inclusion structures in the endothelial cells (Figure A, B). In histological analyses, we found an accumulation of endothelium-associated inflammatory cells, as well as apoptotic bodies, in the heart, small intestine (figure C), and lung (figure D). An accumulation of mononuclear cells was found in the lung, and most of the small pulmonary vessels appeared congested.
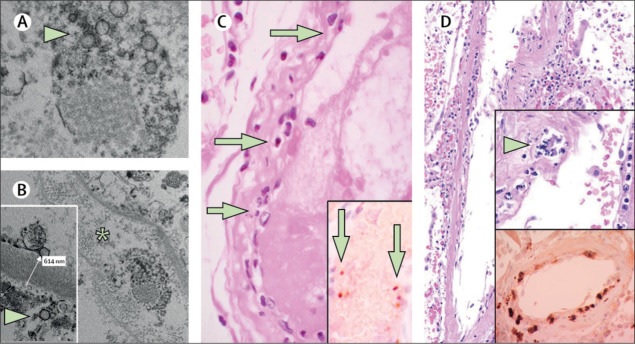
(A, B) Electron microscopy of kidney tissue shows viral inclusion bodies in a peritubular space and viral particles in the endothelial cells of glomerular capillary loops. Aggregates of viral particles (arrow) appear with a dense circular surface and a clear center. The asterisk in panel B marks the peritubular space consistent with the capillary containing viral particles. The inset in panel B shows the glomerular basement membrane with endothelial cells and a viral particle (arrow; approximately 150 nm in diameter). (C) Small bowel resection specimen from patient 3, stained with hematoxylin and eosin. Arrows point to dominant mononuclear cell infiltrates within the intima along the lumen of many vessels. The inset of panel C shows immunohistochemical staining of caspase 3 in small intestine samples from the serial section of the tissue described in panel D. The staining patterns were consistent with the apoptosis of endothelial cells and mononuclear cells observed in sections stained with hematoxylin-eosin, indicating that apoptosis is induced in a substantial proportion of these cells. (D) Post-mortem lung specimen stained with hematoxylin and eosin showed thickened lung septa, including a large arterial vessel with mononuclear and neutrophilic infiltration (arrow in the upper inset). The lower inset shows immunohistochemical staining for caspase 3 in the same lung sample; These staining patterns were consistent with the apoptosis of endothelial cells and mononuclear cells observed in hematoxylin-eosin-stained sections. COVID-19 = coronavirus disease 2019.
Patient 2 was a woman, 58 years old, with diabetes, high blood pressure and obesity. She developed progressive respiratory failure due to COVID-19 and subsequently developed multiple organ failure and required renal replacement therapy.
On day 16, mesenteric ischemia led to removal of the necrotic small intestine. Circulatory failure occurred in the setting of right heart failure secondary to ST-segment elevation myocardial infarction, and cardiac arrest resulted in death.
Postmortem histology revealed lymphocytic endotheliitis in the lung, heart, kidney, and liver, as well as liver cell necrosis. We found histological evidence of myocardial infarction but no sign of lymphocytic myocarditis. Histology of the small intestine showed endotheliitis (endothelialitis) of the submucosal vessels.
Patient 3 was a man, 69 years old, with hypertension who developed respiratory failure as a result of COVID-19 and required mechanical ventilation. Echocardiography showed reduced left ventricular ejection fraction. Circulatory collapse occurred with mesenteric ischemia , and small bowel resection was performed, but the patient survived. Histology of the small bowel resection revealed prominent endotheliitis of the submucosal vessels and apoptotic bodies (Figure C).
We found evidence of direct viral infection of the endothelial cell and diffuse endothelial inflammation. Although the virus uses the ACE2 receptor expressed by pneumocytes in the alveolar epithelial lining to infect the host, thereby causing lung injury, the ACE2 receptor is also widely expressed on endothelial cells, which traverse multiple organs.3
Recruitment of immune cells, either by direct viral infection of the endothelium or mediated by the immune system, can lead to widespread endothelial dysfunction associated with apoptosis (Figure D). The vascular endothelium is an active paracrine, endocrine and autocrine organ that is essential for the regulation of vascular tone and the maintenance of vascular homeostasis.
Endothelial dysfunction is a major determinant of microvascular dysfunction by shifting the vascular balance toward increased vasoconstriction with subsequent organ ischemia, inflammation with associated tissue edema, and a procoagulant state .
Our findings show the presence of viral elements within endothelial cells and an accumulation of inflammatory cells, with evidence of endothelial and inflammatory cell death. These findings suggest that SARS-CoV-2 infection facilitates the induction of endothelitis in various organs as a direct consequence of viral involvement (as observed with the presence of viral bodies) and the host inflammatory response.
Furthermore, the induction of apoptosis and pyroptosis could play an important role in endothelial cell injury in COVID-19 patients. COVID-19 endotheliitis could explain the systemic microcirculatory function in different vascular beds and its clinical sequelae in patients with COVID-19.
This hypothesis provides a rationale for therapies to stabilize the endothelium while addressing viral replication, particularly with anti-inflammatory anti-cytokine medications, ACE inhibitors, and statins 7, 8, 9, 10, 11.
This strategy could be particularly relevant for vulnerable patients with pre-existing endothelial dysfunction , which is associated with male sex, smoking, hypertension, diabetes, obesity, and established cardiovascular disease, all of which are associated with adverse outcomes in COVID-19. 19.
Declaration of conflicts of interest of the authors
ZV and AJF contributed equally as first authors, and RAS, FR and HM contributed equally as last authors. AJF reports rates from Alnylam, Amgen, AstraZeneca, Fresenius, Imedos Systems, Novartis, Pfizer, Roche, Vifor and Zoll, not related to this correspondence. MRM reports consulting relationships with Abbott, Medtronic, Janssen, Mesoblast, Portola, Bayer, NupulseCV, FineHeart, Leviticus, Baim Institute for Clinical Research, Riovant, and Triple Gene, unrelated to this correspondence. FR has been paid for time spent as a committee member for clinical trials, advisory boards, other forms of consulting, and speaking or presentations. These payments were made directly to the University of Zurich and no personal payments were received in connection with these trials or other activities. All other authors declare no competing interests.
















