Rapid Initiation of Saline Nasal Irrigation to Reduce Severity in High-Risk COVID+ Outpatients Summary Aim To determine whether initiating saline nasal irrigation after COVID-19 diagnosis reduces hospitalization and death in high-risk outpatients compared to observational controls, and whether irrigant composition affects severity. Methods Participants aged 55 years and older were enrolled within 24 hours of a COVID-19 + PCR test between September 24 and December 21, 2020. Among the 826 tested, 79 participants were enrolled and randomized to add 2.5 mL of 10% povidone-iodine or 2.5 mL of sodium bicarbonate to 240 mL of isotonic nasal irrigation twice daily for 14 days . The primary outcome was hospitalization or death from COVID-19 within 28 days of enrollment using daily self-report confirmed with phone calls and hospital records, compared to the CDC surveillance data set covering the same time. Secondary outcomes compared symptom resolution with the irrigation additive. Results Seventy-nine high-risk participants (mean [SD] age, 64 [8] years; 36 [46%] female; 71% non-Hispanic white), with a mean BMI of 30.3, were enrolled . Analyzed by intention to treat, by day 28, COVID-19 symptoms resulted in an emergency department visit and no hospitalization in 42 irrigators with alkalinization, one hospitalization in 37 in the povidone-iodine group (1.27%). and no death. Of nearly three million CDC cases, 9.47% were known to be hospitalized, with an additional 1.5% mortality in those without hospitalization data. Age, sex, and percentage with preexisting conditions did not differ significantly by exact binomial test in the CDC data set, while race and reported hospitalization rate did. The overall risk of hospitalization or death (11%) was 8.57 times higher than that of enrolled nasal irrigation participants (SE = 2.74; P = 0.006). Sixty-two participants completed daily surveys (78%), with an average of 1.8 irrigations/day. Eleven reported complaints related to irrigation and four discontinued use. Symptom resolution was more likely for those reporting twice-daily watering (X2 = 8.728, P = 0.0031), regardless of additive.
Conclusion SARS-CoV-2+ participants who initiated nasal irrigation were 8 times less likely to be hospitalized than the national rate. |
Comments
Beginning twice-daily rinsing the mucus-lined nasal cavity with a mild saline solution soon after testing positive for COVID-19 can significantly reduce hospitalization and death, researchers report.
The at-home technique of mixing half a teaspoon of salt and baking soda in a cup of boiled or distilled water and then putting it in a bottle of nasal rinse is a safe, effective and inexpensive way to reduce the risk of serious illness. and death from coronavirus infection that could have a vital impact on public health.
"What we say in the emergency room and surgery is that the solution to contamination is dilution," says Dr. Amy Baxter, an emergency medicine physician at the Medical College of Georgia at Augusta University and corresponding author of the study in Ear, Nose & Throat Journal . “By providing additional hydration to the sinuses, it makes them work better. If you have a contaminant, the more you remove it, the better you can get rid of dirt, viruses, and anything else,” says Ella Baxter.
"We found an 8.5-fold reduction in hospitalizations and no deaths compared to our controls," says lead author Dr. Richard Schwartz, chair of the Department of Emergency Medicine at MCG. "Both of those are pretty important endpoints."
The study appears to be the largest prospective clinical trial of its kind and the older, high-risk population they studied, many of whom had preexisting conditions such as obesity and hypertension, may benefit most from the easy, inexpensive practice, researchers say .
They found that fewer than 1.3% of the 79 study subjects aged 55 and older who enrolled within 24 hours of testing positive for COVID-19 between September 24 and December 21, 2020, experienced hospitalization. No one died.
Among the participants, who were treated at MCG and AU Health System and followed for 28 days, one participant was admitted to the hospital and another went to the emergency room but was not admitted.
By comparison, 9.47% of patients were hospitalized and 1.5% died in a group with similar demographics reported by the Centers for Disease Control and Prevention during the same period, which began about nine months later after SARS-CoV-2 first appeared in the United States.
“The reduction from 11% to 1.3% starting in November 2021 would have corresponded in absolute terms to more than 1 million fewer older Americans requiring admission,” they write. “If confirmed in other studies, the potential reduction in morbidity and mortality worldwide could be profound.”
Schwartz says Baxter brought the idea to him early in the pandemic and liked that it was affordable, easy to use and could impact millions at a time when, like other health care facilities, the Emergency Department at UA Health System was starting to see many SARS-CoV-2 positive patients.
“We were really looking at what options we had available for treatment,” Schwartz says. The first COVID-19 vaccines were administered in December 2020 and the first treatment, the antiviral remdesivir, was approved by the Food and Drug Administration in October 2020.
They knew that the more virus there was in your body, the worse the impact, Baxter says. “One of our thoughts was: If we can eliminate some of the virus within 24 hours of the positive test, then maybe we can reduce the severity of that whole trajectory,” he says, including reducing the likelihood that the virus could enter the lungs, where it was causing permanent damage, often lethal to many.
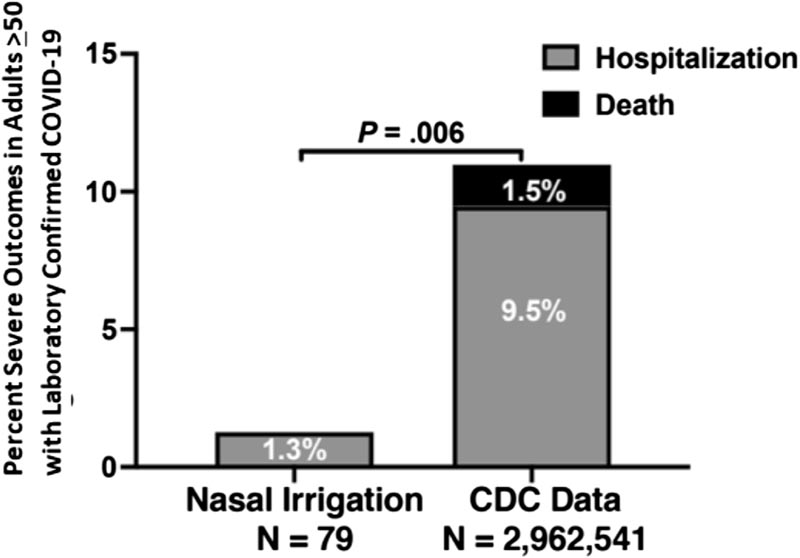 Percentage of serious outcomes in the nasal irrigation group compared to the CDC data set. Percentage of participants older than 55 years in the prospective nasal irrigation group who were hospitalized compared with the number of patients older than 50 years in the CDC national data set reported as hospitalized, or with death reported if the hospitalization information or was missing.
Percentage of serious outcomes in the nasal irrigation group compared to the CDC data set. Percentage of participants older than 55 years in the prospective nasal irrigation group who were hospitalized compared with the number of patients older than 50 years in the CDC national data set reported as hospitalized, or with death reported if the hospitalization information or was missing.














