Summary Impaired olfactory function is a common symptom of COVID-19, but its etiology is unknown. A key question is whether SARS-CoV-2 (CoV-2), the causal agent of COVID-19, affects smell directly, by infecting olfactory sensory neurons or their targets in the olfactory bulb, or indirectly, through disturbance of supporting cells. Here we identify cell types in the olfactory epithelium and olfactory bulb that express SARS-CoV-2 cell entry molecules. Massive sequencing demonstrated that mouse, non-human primate and human olfactory mucosa express two key genes involved in CoV-2 entry, ACE2 and TMPRSS2. However, single-cell sequencing revealed that ACE2 is expressed in supporting cells, stem cells, and perivascular cells, rather than in neurons. Immunostaining confirmed these results and revealed widespread expression of ACE2 protein in the dorsally located olfactory epithelial supported cells and in the pericytes of the olfactory bulb in the mouse. These findings suggest that CoV-2 infection of non-neuronal cell types leads to anosmia and related alterations in odor perception in COVID-19 patients. |
How COVID-19 Causes Loss of Smell Harvard Medical School. By Kevin Jiang
Temporary loss of smell, or anosmia, is the main neurological symptom and one of the earliest and most commonly reported indicators of COVID-19. Studies suggest it is a better predictor of illness than other well-known symptoms such as fever and cough, but the underlying mechanisms of smell loss in COVID-19 patients have been unclear.
Now, an international team of researchers led by neuroscientists at Harvard Medical School has identified the types of olfactory cells in the upper nasal cavity most vulnerable to infection by SARS-CoV-2, the virus that causes COVID-19.
Surprisingly, the sensory neurons that detect and transmit the sense of smell to the brain are not among the vulnerable cell types.
In a report published in Science Advances, the research team found that olfactory sensory neurons do not express the gene that encodes the ACE2 receptor protein, which SARS-CoV-2 uses to enter human cells. Instead, ACE2 is expressed in cells that provide metabolic and structural support to olfactory sensory neurons, as well as certain blood vessel and stem cell populations.
The findings suggest that infection of non-neuronal cell types may be responsible for anosmia in patients with COVID-19 and help inform efforts to better understand disease progression.
"Our findings indicate that the new coronavirus changes the sense of smell in patients not by directly infecting neurons, but by affecting the function of supporting cells," said the study’s senior author, Sandeep Robert Datta, associate professor of neurobiology. at the Blavatnik Institute at HMS.
This implies that, in most cases, SARS-CoV-2 infection is unlikely to permanently damage olfactory neural circuits and cause persistent anosmia, Datta added, a condition that is associated with a variety of mental health problems. and social, particularly depression and anxiety.
"I think it’s good news, because once the infection clears, the olfactory neurons don’t seem to need to be replaced or rebuilt from scratch," he said. "But we need more data and a better understanding of the underlying mechanisms to confirm this conclusion."
Most COVID-19 patients experience some level of anosmia, most often temporary. Analyzes of electronic medical records indicate that patients with COVID-19 are 27 times more likely to have a loss of smell, but only 2.2 to 2.6 times more likely to have fever, cough or shortness of breath, compared with patients without COVID-19.
Some studies have hinted that anosmia in COVID-19 differs from anosmia caused by other viral infections, including other coronaviruses.
For example, COVID-19 patients typically recover their sense of smell over the course of weeks, much faster than the months it can take to recover from anosmia caused by a subset of viral infections known to directly damage olfactory sensory neurons. .
Additionally, many viruses cause a temporary loss of smell by triggering upper respiratory problems, such as nasal congestion. Some COVID-19 patients, however, experience anosmia without nasal obstruction .
Identify the vulnerability
In the current study, Datta and colleagues set out to better understand how the sense of smell is altered in COVID-19 patients by identifying the cell types most vulnerable to SARS-CoV-2 infection.
They began by analyzing existing single-cell sequencing data sets that, in total, cataloged the genes expressed by hundreds of thousands of individual cells in the upper nasal cavities of humans, mice, and non-human primates.
ace2 animation: Rick Groleau
The team focused on the ACE2 gene , widely found in human respiratory tract cells, which encodes the main receptor protein that SARS-CoV-2 targets to enter human cells. They also looked at another gene, TMPRSS2 , which encodes an enzyme thought to be important for SARS-CoV-2 entry into the cell.
Analyzes revealed that both ACE2 and TMPRSS2 are expressed by cells in the olfactory epithelium, a specialized tissue in the roof of the nasal cavity responsible for odor detection that houses olfactory sensory neurons and a variety of supporting cells.
However, none of the genes were expressed by olfactory sensory neurons. Instead, these neurons expressed genes associated with the ability of other coronaviruses to enter cells.
The researchers found that two specific cell types in the olfactory epithelium expressed ACE2 at levels similar to those seen in lower respiratory tract cells, the most common targets of SARS-CoV-2, suggesting a vulnerability to infection.
These included sustainacular cells, which wrap around sensory neurons and are thought to provide structural and metabolic support , and basal cells, which act as stem cells that regenerate the olfactory epithelium after damage. The presence of proteins encoded by both genes in these cells was confirmed by immunostaining.
In additional experiments, the researchers found that olfactory epithelial stem cells expressed the ACE2 protein at higher levels after artificially induced damage, compared to resting stem cells. This may suggest additional vulnerability to SARS-CoV-2, but it is unclear whether this is important for the clinical course of anosmia in COVID-19 patients, the authors said.
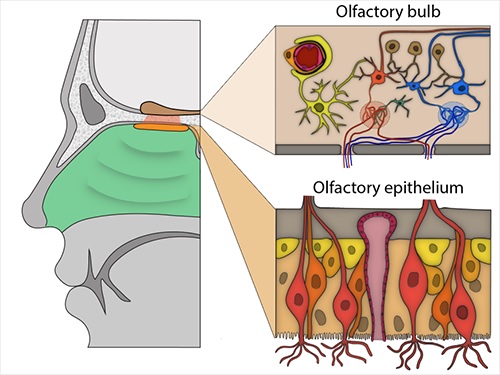
Olfactory bulb and epithelium. Important cells: Top right: A pericyte (light orange) wraps around a blood vessel (red). Bottom right: olfactory sensory neurons (light red, orange) surrounded by Sustainacular cells (tan) and basal cells (light yellow and orange). Image: Brann et. al., 2020.
Datta and his colleagues also analyzed gene expression in nearly 50,000 individual cells in the mouse olfactory bulb, the structure in the forebrain that receives signals from olfactory sensory neurons and is responsible for initial odor processing.
Olfactory bulb neurons did not express ACE2.
The gene and associated protein were present only in blood vessel cells, particularly pericytes, which are involved in regulating blood pressure, maintaining the blood-brain barrier, and inflammatory responses. No olfactory bulb cell type expressed the TMPRSS2 gene.
Clue of loss of smell
Together, these data suggest that COVID-19-related anosmia may arise from a temporary loss of supporting cell function in the olfactory epithelium, which indirectly causes changes in olfactory sensory neurons, the authors said.
"However, we still don’t fully understand what those changes are," Datta said. "Sustacular cells have been largely ignored, and it seems we need to pay attention to them, similar to how we have a growing appreciation for the critical role that glial cells play in the brain."
The findings also offer interesting clues about the neurological problems associated with COVID-19. The observations are consistent with the hypothesis that SARS-CoV-2 does not directly infect neurons, but rather may interfere with brain function by affecting vascular cells in the nervous system, the authors said. This requires more research to verify, they added.
The study results now help accelerate efforts to better understand smell loss in COVID-19 patients, which in turn could lead to treatments for anosmia and the development of better smell-based diagnostics for the disease.
"Anosmia seems like a curious phenomenon, but it can be devastating for the small fraction of people in whom it persists," Datta said. “It can have serious psychological consequences and could be a major public health problem if we have a growing population with permanent loss of smell.”
The team also hopes the data can help pave the way for questions about disease progression, such as whether the nose acts as a reservoir for SARS-CoV-2 . Such efforts will require studies in facilities that allow experiments with live coronaviruses and analysis of human autopsy data, the authors said, which are still difficult to obtain. However, the collaborative spirit of pandemic-era scientific research demands optimism.
“We started this work because my lab had a couple of data sets ready to analyze when the pandemic hit, and we published an initial preprint,” Datta said. “What happened after that was amazing, researchers from all over the world offered to share and merge their data with us in a kind of makeshift global consortium. “This was a true collaborative achievement.”
The study’s first co-authors are David Brann, Tatsuya Tsukahara and Caleb Weinreb. Other authors include Marcela Lipovsek, Koen Van den Berge, Boying Gong, Rebecca Chance, Iain Macaulay, Hsin-jung Chou, Russell Fletcher, Diya Das, Kelly Street, Hector Roux de Bezieux, Yoon-Gi Choi, Davide Risso, Sandrine Dudoit , Elizabeth Purdom, Jonathan Mill, Ralph Abi Hachem, Hiroaki Matsunami, Darren Logan, Bradley Goldstein, Matthew Grubb and John Ngai.
The study was supported by grants from the National Institutes of Health (grants RO11DC016222 and U19 NS112953) and the Simons Collaboration on the Global Brain. Additional funding information can be found in the full text of the document. DOI: 10.1126/sciadv.abc1564