Key points What are the temporal associations between higher body mass index (BMI) and chronic inflammation and/or hyperinsulinemia? Findings In this systematic review and meta-analysis of 5603 participants in 112 cohorts from 60 studies, the association between period 1 (earlier) fasting insulin levels and period 2 (later) BMI was positive and significant: for each unit change of SD in period 1 insulin level, there was a consequent associated change of 0.26 SD units in period 2 BMI. Meaning These findings suggest that the adverse consequences currently attributed to obesity could be attributed to hyperinsulinemia (or another proximate factor). |
Obesity is associated with a number of chronic non-communicable diseases (NCDs), such as type 2 diabetes, coronary heart disease, chronic kidney disease and asthma. Although obesity is also hypothesized to cause premature death, this association does not meet several of the Bradford Hill criteria for causality.
- First, the possible attributable risk of death is small (<5%).
- Second, the dose-response gradient between body mass index (BMI) and mortality is U-shaped with overweight (and possibly obesity level I) as minimums.
- Third, evidence from animal models comes primarily from mice that have been fed high-fat diets; Unlike humans, these animals typically did not have fat as part of their typical diet and therefore the experiments are not potentially analogous to those in humans.
- Fourth, the evidence that people with obesity live longer than their lean counterparts in populations with acute or chronic conditions and older age is remarkably consistent. Therefore, it is possible that instead of being a risk factor for NCDs, obesity is actually a protective factor. response to the development of the disease.
Putative links between obesity and adverse outcomes are often attributed to two potential mediators: chronic inflammation and hyperinsulinemia.
These characteristics have been associated with several NCDs, including obesity, as well as type 2 diabetes, cardiovascular disease, and chronic kidney disease. Existing data on the association of obesity with chronic inflammation and/or hyperinsulinemia are mainly cross-sectional, making it difficult to confirm the direction of any causality.
This systematic review and meta-analysis summarizes the evidence on the temporality of the association between higher BMI and chronic inflammation and/or hyperinsulinemia. We hypothesized that changes in chronic inflammation and hyperinsulinemia would precede changes in higher BMI.
Importance
Obesity is associated with a number of chronic non-communicable diseases and is thought to cause premature death.
Aim
To summarize the evidence on the temporality of the association between higher body mass index (BMI) and 2 potential mediators: chronic inflammation and hyperinsulinemia.
We searched the data sources MEDLINE (1946 to August 20, 2019) and Embase (1974 to August 19, 2019), although only studies published in 2018 were included due to the high volume of results. Data analysis was carried out between January 2020 and October 2020.
Selection of studies and measures
Longitudinal studies and randomized clinical trials that measured fasting insulin level and/or a marker of inflammation and BMI with at least 3 proportional time points were selected.
Data extraction and synthesis
The slopes of these markers were calculated between time points and standardized. Standardized slopes were meta-regressed in later periods (period 2) with standardized slopes in earlier periods (period 1). Evidence-based items that could indicate a risk of bias were assessed.
Results
From 1865 records, 60 eligible studies were identified with 112 cohorts of 5603 participants. Most standardized slopes were negative, meaning that participants in most studies experienced decreases in BMI, fasting insulin level, and C-reactive protein level.
The association between period 1 fasting insulin level and period 2 BMI was positive and significant (β = 0.26; 95% CI, 0.13-0.38; I2 = 79%): for each unit change in SD in period 1 insulin level, there was a subsequent associated change of 0.26 SD units in period 2 BMI.
The association of period 1 fasting insulin level with period 2 BMI remained significant when period 1 C-reactive protein level was added to the model (β = 0.57; 95% CI, 0.27- 0.86).
In this bivariate model, period 1 C-reactive protein level was not significantly associated with period 2 BMI (β = –0.07; 95% CI, –0.42 to 0.29; I2 = 81% ).
Bubble plot of temporal associations between changes in period 1 and period 2
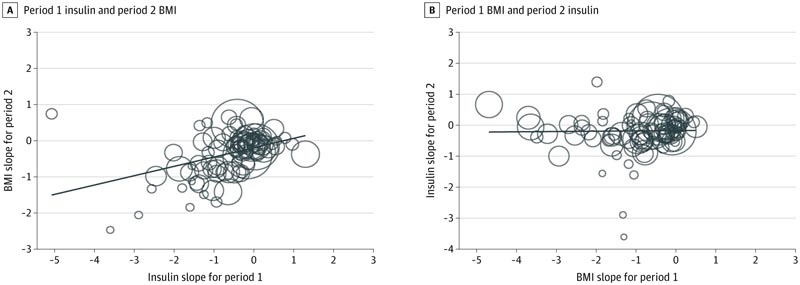
A, The period 2 change in body mass index (BMI) (or standardized slope) is regressed to the period 1 change in insulin. B, The change in insulin in period 2 regresses to the change in BMI in period 1. The flat trend line in panel B suggests that there is no association between the change in period 1 BMI and the change in insulin of period 2. The diagonal trend line in panel A supports a positive, temporal association between period 1 change in insulin and period 2 change in BMI. The size of the circles is based on the inverse of the SE of each cohort.
Conclusions and relevance In this meta-analysis, the temporal sequencing finding (in which changes in fasting insulin level precede changes in weight) is not consistent with the claim that obesity causes chronic noncommunicable diseases and premature death by increasing fasting insulin levels. |
Discussion
This systematic review and meta-analysis suggests that decreases in fasting insulin are more likely to precede decreases in weight than decreases in weight to precede decreasing levels in fasting insulin.
After accounting for the association between previous fasting insulin levels and the subsequent likelihood of weight gain, there was no evidence that inflammation preceded subsequent weight gain.
This temporal sequence (in which changes in fasting insulin precede changes in weight) is not consistent with the claim that obesity causes NCDs and premature death by increasing fasting insulin levels.
Support from other studies
In patients with type 2 diabetes, RCTs have found that the introduction of insulin and exogenous sulfonylureas (which increase endogenous insulin production) compared to lower doses or no drug treatment results in weight gains. Some patients with type 1 diabetes deliberately skip or reduce their insulin injections. Similarly, reports following bariatric surgery consistently indicate that insulin levels decline before weight in patients undergoing bariatric surgery.
Therefore, the finding that changes in insulin levels tend to precede changes in weight and not the other way around was previously demonstrated in 3 different scenarios. To our knowledge, there is no clinical evidence demonstrating that weight gain or loss precedes increases or decreases in endogenous insulin.
Importance of the findings
Obesity as a cause of premature death does not meet several of the Bradford Hill criteria for causality: the strength of the association is small; the consistency of the effect in older and/or sick populations favors obesity; and the biological gradient is U-shaped, with overweight and level 1 obesity associated with the lowest risk; and if hyperinsulinemia is considered the mediator, then the temporal sequence is incorrect.
Insulin resistance , a cause and consequence of hyperinsulinemia, leads to type 2 diabetes and is associated with other adverse outcomes, such as myocardial infarction, chronic lung disease, and some cancers, and may also be implicated in diabetic nephropathy.
Despite the 3 scenarios described above, it is commonly believed that obesity leads to hyperinsulinemia. If the opposite is true and hyperinsulinemia actually leads to obesity and its putative adverse consequences, then weight loss without concomitant insulin decreases (e.g., Liposuction) would not be expected to address these adverse consequences. Furthermore, weight loss would not address the risk in people with so-called metabolically healthy obesity, that is, those without insulin resistance.
It is interesting to note that insulin resistance is also present in lean people , particularly men and people of Asian descent. These 2 groups have a higher risk of type 2 diabetes and cardiovascular disease, but are more likely to be thin than women and people of non-Asian descent.
These observations are consistent with the hypothesis that hyperinsulinemia, rather than obesity, is driving adverse outcomes in this population.
| We speculate that the ability to store the byproducts of excess glucose by increasing the size of fat cells (manifesting as obesity) could delay the onset of type 2 diabetes and its consequences in some individuals, thus explaining the so-called obesity paradox. lower mortality. among people with obesity. This idea, while not new, fits better with emerging evidence. |
If this speculation is correct, evaluating the ability to store such byproducts at the individual level may be a useful step toward personalized medicine.
Although hyperinsulinemia per se may not be the causal agent leading to adverse outcomes (but rather a marker for another, more proximal factor), this would not change the lack of support for recommending weight loss among people with obesity. Rather, other markers should be investigated that, although correlated with obesity, are more strongly associated with premature mortality because they also exist in lean individuals.
Therapies that reduce insulin levels (eg, moderate diets with fewer simple carbohydrates and metformin) may be sustainable if an intermediate marker other than weight is sought.
As the prevalence of obesity continues to increase worldwide, additional studies are urgently needed to confirm this hypothesis, especially since public health campaigns promoting weight loss are ineffective and lead to stigma100 among people with obesity. obesity.
Limitations
This study has limitations. First, the identified studies largely enrolled chronically obese participants undergoing weight loss interventions and the measures of interest (e.g., weight, insulin level, and CRP level) mostly decreased. The findings are limited to those people who lose weight and, given the findings from the bariatric subgroup analysis, are likely driven by rapid decreases in circulating insulin levels.
Second, the included populations mostly had mean baseline CRP levels between 1 and 10 mg/L suggesting a low degree of chronic inflammation normally associated with atherosclerosis and insulin resistance. Several studies90,101-104 have highlighted a group of people characterized by CRP levels consistently greater than 10 mg/L. Although this higher degree of chronic inflammation is associated with obesity, few participants had insulin resistance, which suggests a different grouping.
Third, this meta-analysis used summary-level data rather than individual patient data and is therefore vulnerable to fallacy. A prospective cohort study designed for weight loss or gain with very frequent measurements in a diverse population would provide a stronger form of evidence.
Fourth, the review was limited to studies published in 2018, and many of the studies indicate a significant risk of bias with respect to their stated objectives. However, neither study was designed to measure temporal associations between the measures of interest, so these limitations in study conduct would not necessarily have led to bias with respect to the findings. Although the search was limited to a single publication year (2018) to reduce the workload associated with this review, there is no reason to expect this year’s data to differ from data published before or after.
Conclusions
|
















