The National Center for Cardiovascular Research (CNIC), in collaboration with scientists from Spain and abroad, has developed a new diagnostic tool that allows simultaneous evaluation of the electrical and mechanical (contractile) activity of the heart.
Summary Non-invasive electromechanical evaluation during atrial fibrillation identifies underlying alterations of atrial myopathy with early prognostic value Electromechanical characterization during atrial fibrillation (AF) remains a major gap in the understanding of AF-related atrial myopathy . This study reports mechanistic insights into the electromechanical remodeling process associated with AF progression and further demonstrates its prognostic value in the clinic. In pigs, sequential electromechanical evaluation during AF progression shows a progressive decrease in mechanical activity and an early dissociation from its electrical counterpart. Atrial tissue samples from animals with AF reveal an abnormal increase in cardiomyocyte death and alterations in calcium-handling proteins. High-throughput quantitative proteomics and immunoblot analyzes at different stages of AF progression identify downregulation of contractile proteins and progressive increase in atrial fibrosis. Furthermore, advanced optical mapping techniques, applied to whole-heart preparations during AF, demonstrate that AF-related remodeling decreases the frequency threshold for dissociation between transmembrane voltage signals and intracellular calcium transients compared with healthy controls. Single-cell simulations of human atrial cardiomyocytes also confirm the experimental results. In patients, noninvasive assessment of the atrial electromechanical relationship further demonstrates that atrial electromechanical dissociation is an early prognostic indicator for acute and long-term rhythm control. They demonstrate that AF-related remodeling decreases the frequency threshold for dissociation between transmembrane voltage signals and intracellular calcium transients compared to healthy controls. |
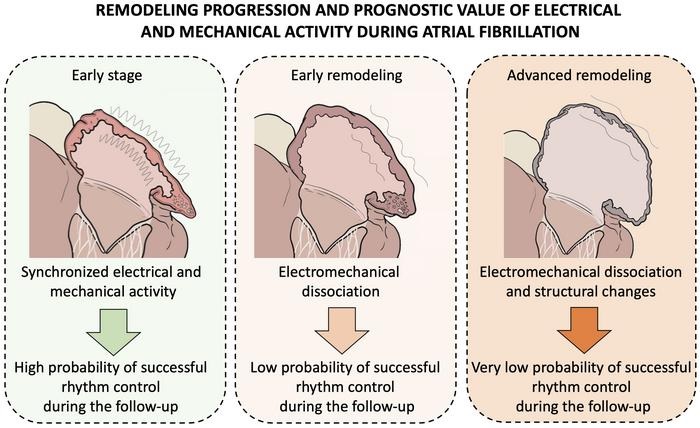
Figure : Schematic representation of the progression of electromechanical remodeling of the atria of the heart during atrial fibrillation, indicating at each stage of the disease the probability of restoring normal rhythm after an uninterrupted episode of atrial fibrillation.
Comments
A multidisciplinary study led by scientists from the National Center for Cardiovascular Research (CNIC) presents a new method to evaluate the structural and electrophysiological changes, called atrial remodeling , that occurs in the heart of patients with atrial fibrillation, one of the most frequent forms of arrhythmia. The new diagnostic method is based on the simultaneous evaluation of electrical and mechanical (contractile) activity in the atria of the heart during atrial fibrillation. The study is published in Nature Communications (DOI 10.1038/s41467-023-40196-y).
The leader of the study, David Filgueiras, explained that, until now, “this was an unmet challenge”, because, on the one hand, “the available technology did not allow integrating both types of information to provide a more complete evaluation”, and on the other On the other hand, “during atrial fibrillation, the contractile movements of the atria are of low intensity and measuring them is a technical challenge.” Atrial fibrillation is an irregular and often very rapid heartbeat that can cause blood clots to form in the heart, increasing the risk of stroke, heart failure, and related complications. The condition of atrial fibrillation affects about 10 million people in Europe and around 700,000 in Spain.
Atrial fibrillation is currently classified based on how long the patient has been in arrhythmia. However, explains Dr. Filgueiras, who leads the Advanced Development in Arrhythmia Mechanisms and Therapy group at the CNIC and is a cardiologist at the Health Research Institute of the San Carlos Clinical Hospital (IdISSC), “this temporal classification does not provide information about the status of a patient or the degree of atrial remodeling , an especially important parameter in the first months of the condition, when the underlying disease processes can progress at different rates.
According to Dr. Filgueiras, “the importance of this new diagnostic method is its ability to provide a personalized assessment of the degree of atrial remodeling of an individual patient, regardless of clinical classification based on temporal criteria.”
The first three authors of the study are Daniel Enríquez Vázquez, from the University Hospital Complex of A Coruña and member of the Spanish Cardiovascular Research Network (CIBERCV), and CNIC scientists Jorge G. Quintanilla and Alba García Escolano. Dr. Enríquez Vázquez highlighted that “at a clinical level, the results show that electromechanical dissociation in patients with atrial fibrillation is a solid indicator of disease progression and the need to take urgent measures to return these patients to a normal rhythm.” efficiently and stably. manner."
The team led by Dr. Filgueiras worked in collaboration with colleagues from the San Carlos Clinical Hospital, the Central University Hospital of Asturias, the Hospital de la Santa Creu i Sant Pau, the University Hospital Complex of A Coruña, the Complutense University of Madrid, the Polytechnic University of Madrid, the Autonomous University of Barcelona, the University of Connecticut and the CIBERCV. Over the past 10 years, this team of national and international experts worked together to integrate electrical and mechanical cardiac data to enable personalized characterization of the status of pathological changes associated with atrial fibrillation progression.
This diverse team was able to achieve this by using a multidisciplinary approach.
In the first phase, engineers and physicists designed the most appropriate strategy to integrate electrical and mechanical data . The solution they found was to measure mechanical activity using Doppler imaging, a non-invasive method that provides information about atrial tissue movements, and electrical activity using surface electrocardiography.
Both approaches are easy to implement in the clinic because they are non-invasive and can be performed during a transthoracic ultrasound examination, a routine study of the shape and function of the heart and some of its internal structures.
Experts in biology, biotechnology, biochemistry and biomedical engineering participated in the second phase, working together with the CNIC Proteomics Unit and clinical cardiologists. Experimental studies performed in this phase correlated the information obtained with the new approach with the underlying pathological changes in the auricular tissue. This information was used to develop new advanced mapping techniques and computer simulations to reveal the mechanisms underlying electrical and mechanical remodeling during AF progression.
The final phase was a prospective multicenter study of 83 patients at an early stage in the development of atrial fibrillation, to determine the prognostic value of simultaneous electrical and mechanical evaluation of the atria in patients with this type of arrhythmia.
Experimental and clinical findings revealed an imbalance between electrical and mechanical (contractile) activation in the atria in the early stages of the disease. This causes the two parameters to dissociate , so that contractile activation cannot keep pace with electrical activation, a phenomenon researchers call atrial electromechanical dissociation . The rhythm of this dissociation is specific to the individual patient, although in the absence of restoration of a normal rhythm it is usually observed within the first 2-3 months after an uninterrupted episode of atrial fibrillation.
A key advantage of the new approach is that atrial electromechanical dissociation is identified before the appearance of overt clinical signs of structural atrial remodeling . “The use of this new diagnostic approach allows early characterization of the underlying remodeling in patients with atrial fibrillation,” said Dr. Filgueiras. “The study demonstrates that it is possible to integrate electrical and mechanical data from the atria of patients with atrial fibrillation to obtain personalized prognostic information about the clinical progression of the disease.”
Nicasio Pérez Castellano, from the IdISSC, and David Calvo Cuervo, from the Central Hospital of Asturias and currently a member of the IdISSC, highlighted that, “in addition to the results obtained, a great strength of this new approach is its non-invasive nature, which greatly facilitates management.” of patients with atrial fibrillation.
Julián Pérez Villacastín, clinical collaborator of the study at the Hospital Clínico San Carlos, CIBERCV and currently president of the Spanish Society of Cardiology, added that “this research and its clinical implementation will allow increasingly personalized management of patients with atrial fibrillation.”
The study was funded by the European Union H2020 Program (Grant Agreement No. 965286), the Ministry of Science and Innovation (PID2019-109329RB-I00 and PGC2018-097019-B-I00), the Carlos III Health Institute (European Health Research Fund PRB3 (PT17/0019/0003-ISCIII-SGEFI/FEDER, ProteoRed), Interhospital Fund for Cardiovascular Research, CIBERCV, Fundación Salud 2000 and Fundación La Caixa (project code HR17-00247).















