Summary Although it has been almost a year since the outbreak of Coronavirus Disease 2019 (COVID-19) and there have been promising reports of vaccines, we still have a long way to go until these measures are available to everyone. Furthermore, the most appropriate corticosteroid and dose in the treatment of COVID-19 remain uncertain. We conducted a study to evaluate the effectiveness of methylprednisolone versus dexamethasone treatment for hospitalized patients with COVID-19. Methods In this triple-blind prospective randomized controlled trial, we enrolled 86 patients hospitalized with COVID-19 from August to November 2020, in Shiraz, Iran. Patients were randomly assigned into two groups to receive methylprednisolone (2 mg/kg/day; intervention group) or dexamethasone (6 mg/day; control group). Data were evaluated based on a 9-point WHO ordinal scale ranging from not infected (point 0) to death (point 8). Results There were no significant differences between the groups at admission. However, the intervention group demonstrated significantly better clinical status compared to the control group on day 5 (4.02 vs. 5.21, p = 0.002) and day 10 (2.90 vs. 4.71, p = 0.001) of admission. . There was also a significant difference in the overall mean score between the intervention group and the control group (3.909 vs. 4.873 respectively, p = 0.004). The mean length of hospital stay was 7.43 ± 3.64 and 10.52 ± 5.47 days in the intervention and control groups, respectively (p = 0.015). The need for a ventilator was significantly lower in the intervention group than in the control group (18.2% vs 38.1% p = 0.040). Conclusion In hospitalized patients suffering from COVID-19 pneumonia, administration of 2 mg/kg per day of intravenous methylprednisolone compared to treatment with 6 mg/day of dexamethasone, led to a reduction in the length of hospital stay, the need for mechanical ventilation and improved clinical status on days 5 and 10. |
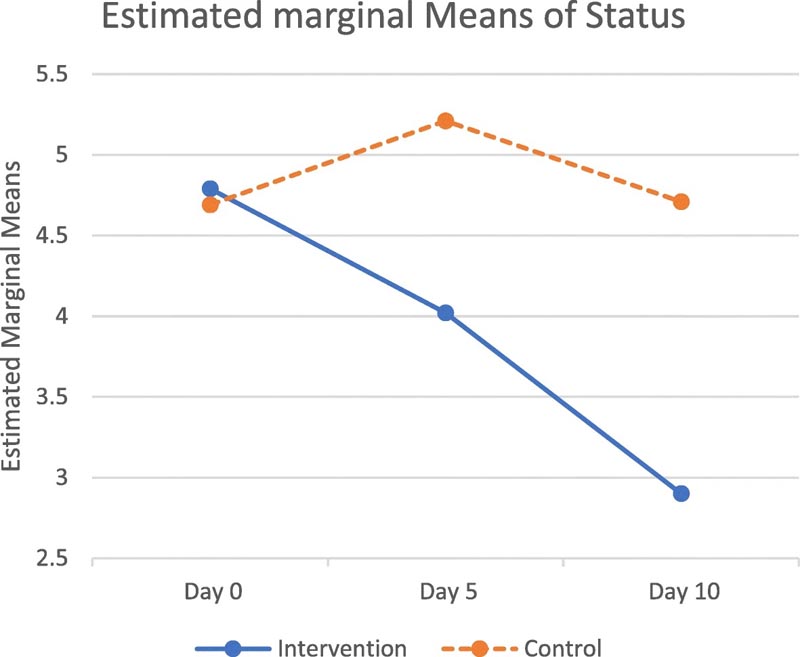
Diagram of the clinical status in the intervention (methyl-prednisolone) and control groups
Discussion
In our study, both treated groups received corticosteroids (the control group received dexamethasone); However, those who received methylprednisolone ended up having better outcomes and less dependence on mechanical ventilation. These data suggest that better penetration of methylprednisolone into the lungs compared to dexamethasone may have led to the better outcomes observed; as also suggested by multiple studies that demonstrate better penetration of methylprednisolone into lung tissue compared to other corticosteroids.
Instead, the differences found can be explained by the relatively higher dose of corticosteroid, given that the estimated 6 mg of dexamethasone per day is approximately equivalent to 32 mg of methylprednisolone. This suggests that the control group was receiving approximately 0.5 mg/kg per day based on a standard 70 kg male and therefore the methylprednisolone group received a more potent dose . Whether due to differences in dosage or medication, 2 mg/kg methylprednisolone led to better outcomes in hypoxic hospitalized COVID-19 patients compared to 6 mg/day dexamethasone.
Although treatment of patients suffering from COVID-19 with glucocorticoids may have some complications, such as superimposed infection, immunosuppression, and hyperglycemia, recent studies did not report significant complications in the course of their study. However, hyperglycemia was more common in those receiving methylprednisolone, managed without substantial complications. Furthermore, it is suggested that the full dose of appropriate antibiotic therapy and immune regulators such as human immunoglobulin should be used to improve the immunity of patients in cases with complications.
This study had several limitations , including small sample size in each group and limited data on complications, laboratory data, and CT scan characteristics. Given the limitations of the study, further randomized controlled trials with larger sample sizes and subsequent follow-ups are required to evaluate the beneficial effect of methylprednisolone in patients with COVID-19 pneumonia.
Comments
Dexamethasone prevents some deaths among hypoxemic patients with COVID-19 (NEJM JW Gen Med Aug 15, 2020 and N Engl J Med 2021;384:693). However, methylprednisolone reaches higher concentrations in lung tissue than dexamethasone, raising questions about whether it would be more effective.
Iranian researchers randomly assigned 86 adults with confirmed SARS-CoV-2 infection who were hospitalized (with oxygen saturation ≤92% on room air) to receive intravenous methylprednisolone (daily dose of 2 mg/kg tapered after 5 days). ; total dose, 10 days) or intravenous dexamethasone (6 mg daily for 10 days).
Patients and investigators were blinded to drug assignments.
According to the WHO Ordinal Scale of Clinical Improvement (OSCI), patients receiving methylprednisolone had significantly greater clinical improvement than patients receiving dexamethasone.
Methylprednisolone patients also had significantly lower ventilator needs (18% vs. 38%; number needed to treat, 5), significantly shorter hospital stay (3 fewer days), and a trend toward lower mortality (19% vs. 38). %; NNT, 6; P = 0.076), compared with dexamethasone patients.
Although it is unclear whether the results of this study are due to the type of steroid and its enhanced lung penetration or the higher relative dose of methylprednisolone prescribed, this approach could be considered in patients with severe COVID-19.
We are now seeing fewer and fewer patients admitted with severe COVID-19, but some patients with severe disease could still benefit from this adjusted approach to steroid therapy. Although it is unclear whether the results of this study are due to the type of steroid and its enhanced lung penetration or the higher relative dose of methylprednisolone prescribed, this approach could be considered in patients with severe COVID-19.
















