| Highlights |
|
| 1. Introduction |
Cardiovascular disease (CVD) represents the main cause of morbidity and mortality worldwide. Global disability trends adjusted for years of life and years of life lost almost doubled. High blood pressure (HTN) is the main modifiable risk factor, responsible for structural and/or functional changes in main organs.
The presence of hypertensive-mediated organ damage reflects the severity and practical burden of HTN in such patients, in whom their total CV risk significantly increases. There is a strong epidemiological relationship between chronic HTN, CAD and cerebrovascular disease.
There is a linear correlation between blood pressure (BP) levels and the risk of CAD, while almost 25% of the risk of myocardial infarction attributable to the population may be represented by HTN. Likewise, HTN is the prevalent risk factor for cerebrovascular accident (CVA) presented in almost two thirds of these patients. Therefore, very often doctors will have to evaluate and treat patients with HTN and CAD or cerebrovascular diseases.
Treatment of HTN is imperative since reducing BP translates into an improvement in CV morbidity and mortality. Each systolic BP (SBP) reduction of 10 mmHg decreases the risk of CV events by approximately 20%. However, how low should BP be in patients with chronic coronary or cerebrovascular diseases?
In the last 3 decades, many researchers have joined forces as well as meta-analyses focused on the dangers of reducing BP below a certain threshold, especially in patients with CVD and stroke. Despite this, current ACC/AHA guidelines for the treatment of HTN recommend more aggressive BP reduction, a strategy driven primarily by the results of the SPRINT trial. In contrast, the ESC/ESH guidelines adopted a more conservative strategy, especially in patients in whom intensive BP reduction may be harmful.
| 2. Lower blood pressure thresholds in patients with chronic coronary and/or cerebrovascular diseases |
According to the ACC/AHA guidelines for the management of hypertensive patients with clinical CAD or stroke), the recommended BP goal is <130/80 mmHg, with levels <120/80 mmHg considered normal values. Furthermore, these patients should begin antihypertensive treatment and lifestyle modifications when BP levels are ≥130/80 mmHg. On the contrary, the ESC/ESH and the International Society of Hypertension (ISH) guidelines target BP in patients <65 years of age, a value <130/80 mmHg and not a value <120/70 mmHg. On the other hand, antihypertensive treatment should be started when BP levels are ≥140/90 mmHg.
The difference between these guidelines with respect to the lower BP threshold lies in the fact that the ESC/ESH and ISH guidelines respect the possible existence of the J-curve phenomenon (the ACC/AHA guidelines do not have any lower BP threshold). BP that must be respected in hypertensive patients with CAD). The ACC/AHA guidelines based their recommendation primarily on the results of the SPRINT trial and two meta-analyses.
In the SPRINT trial, intensive BP treatment targeting SBP <120 mmHg, compared with <140 mmHg, resulted in lower rates of deaths and nonfatal major CV events, and death from any cause (however, not no decrease in myocardial infarction was noted while BP measurement was neglected). On the other hand, the results of the 2 meta-analyses showed that achieving BP levels <130/80 mmHg was safe, with an improvement in CV morbidity and mortality.
| 3. Coronary flow and blood pressure levels |
Coronary blood flow occurs primarily in diastole and subendocardial flow is exclusively a diastolic event. The factors that affect coronary flow are mainly coronary flow resistance, coronary perfusion pressure (pressure gradient between the coronary arteries and the right atrium), and the duration of diastole.
When myocardial oxygen demands increase consumption, coronary resistance decreases to increase coronary flow. However, to have adequate myocardial perfusion, the coronary perfusion pressure must be at least 50 and 65 mmHg, assuming that the left ventricular diastolic pressure is within normal limits (5-12 mmHg). It should be noted that in patients with CAD, coronary perfusion pressure is related to the diastolic pressure of the coronary artery, distal to significant coronary obstruction (which is lower than the aortic diastolic pressure).
| 4. Blood pressure goal in patients with coronary artery disease |
There is overwhelming evidence, especially in high-risk patients, that lowering BP below a certain threshold increases the risk of a subsequent CV event. In several studies, such as ONTARGET (treatment with telmisartan, ramipril or both, of patients at high risk of vascular events), and VALUE (hypertensives with high CV risk treated with valsartan or amlodipine) in high-risk patients, as well as In the INVEST revised (review of findings from the International Verapamil SR-Trandolapril Study) and the TNT (Treating to New Targets) studies of hypertensive patients with CAD, there is a J-shaped relationship between BP levels and CV events.
In these studies, BP levels <120 and/or 70 mmHg significantly increased CV risk (although in the INVEST study it is likely that rather than an increase in the event rate, there was a decrease in the extent of benefit) . On the other hand, data from the Clarify registry with 22,672 hypertensive patients with stable CAD showed that BP <120 and/or 60 mmHg were associated with adverse CV outcomes, including mortality. On the other hand, coronary perfusion pressure must be maintained to have adequate myocardial perfusion.
There are studies that state that in patients with CAD, with or without revascularization, diastolic BP levels <70 mmHg led to a marked reduction in coronary blood flow, a phenomenon that was much more intense in patients with left ventricular hypertrophy, a very serious condition. common in hypertensive patients.
Therefore, it is not surprising that in hypertensive patients with CAD, decreased BP levels <70 mmHg were also independently associated with progressive myocardial damage, expressed by increased ultrasensitive troponin-T. On the other hand, in the SPRINT trial, intensive BP treatment targeting SBP <120 mmHg, compared with <140 mmHg, did not improve the prognosis of hypertensive patients with a history of CVD. Likewise, a subgroup analysis of the SPRINT study (1,206 participants with CAD and 8,127 participants without CAD) found that intensive BP treatment decreased the risk of major CV events in participants without CAD, but not in those with CAD.
For participants with CAD, intensive BP treatment was associated with a reduced risk of death from all causes, but did not affect other clinical outcomes, compared with standard BP treatment. Furthermore, in the ACCORD study, patients with type 2 diabetes and a history of CV events, targeting SBP <120 mm Hg, compared with <140 mm Hg, did not reduce the rate of a composite outcome of CV events fatal and non-fatal. According to the authors, the lack of randomized controlled studies (RCTs) designed to evaluate the existence of the J curve phenomenon is regrettable.
Low BP levels may be related to poor clinical conditions (neoplasia, infection, malnutrition, and heart failure) and therefore present a higher rate of events. In the INDANA (Individual Antihypertensive Data Analysis) study, a meta-analysis that included 40,233 hypertensive patients (mean follow-up, 3.9 years), the authors concluded that poor health conditions were responsible for low BP levels. , with increased risk of death.
The increased risk of events was not related to antihypertensive treatment nor was it specific to BP-related vascular events. Furthermore, in a recent meta-analysis, the authors found that in preventing CV death, more intensive BP treatment was superior to the less intensive control strategy, although these results are still inconclusive.
Although there are contradictory results regarding the existence of the J-curve phenomenon, in patients with CAD there is no evidence that reducing BP levels <120/70 mmHg is beneficial. Therefore, the threshold of SBP <120 mmHg adopted by several algorithms for the treatment of stable angina. Antianginal medications were used in this study, since beta blockers, calcium channel blockers or nitrates also have antihypertensive effects and, in these patients, justify the BP thresholds <120/70 mmHg of the ESC/ESH guidelines. for the management of HTN.
| 5 . Cerebral blood flow and blood pressure levels |
Cerebral vessels are very vulnerable to the effects of higher BP and both high systolic and diastolic hypertension are risk factors for ischemic stroke (CVA) and hemorrhagic stroke.
Autoregulation of cerebral circulation makes it possible to maintain cerebral blood flow at stable levels, despite changes in BP in the range of 60 to 150 mmHg. However, sustained HTN causes marked adaptive changes in cerebral circulation, including increased resistance of cerebral vessels and modifications of the physiological autoregulation mechanism.
HTN modifies the autoregulation of cerebral blood flow, changing the lower and upper limits of the autoregulation capacity towards higher BP values. Therefore, hypertensive patients may be especially vulnerable to episodes of HBP, which may play a role in the development of silent cerebrovascular damage.
The effect of elevated BP on small vessels is well known, with vascular remodeling occurring in cerebral blood vessels during chronic HTN. This structure alters self-regulation, playing a role in the development of silent brain disease, which includes white matter lesions, microbleeds and lacunar infarcts, evolving to clinical outcomes such as stroke and dementia.
| 6. Target blood pressure to prevent primary strokes |
Prospective studies have shown a continuous, strong, and independent positive relationship between BP levels and CVD. This applies to both SBP and diastolic BP. Furthermore, pooled evidence from prospective cohort studies suggests that the minimum risk BP for CVD could be a SBP of 110 mmHg to 115 mmHg.
A meta-analysis of RCTs including hundreds of thousands of patients has shown that a reduction of 10 mmHg in SBP or 5 mmHg in diastolic BP is associated with significant reductions of almost 20% in all major CV events, 35 % reduction in stroke and 10-15% in overall mortality. These relative risk reductions are consistent regardless of baseline BP within the hypertensive range, CV risk level, comorbidities (eg, diabetes, chronic kidney disease), age, sex, and ethnicity.
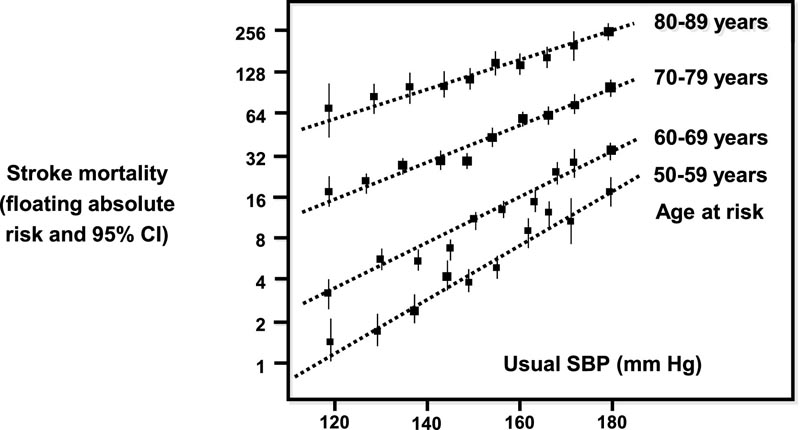
Figure 1 Stroke mortality rate in each decade versus usual SBP at the beginning of that decade (floating absolute risk and 95% CI). Modified and adapted from reference.
To achieve target levels <140/90 mmHg in the office, the ESH/ESC hypertension guidelines recommend a target BP <140/90 mmHg, regardless of the number of comorbidities and the level of CV risk. At that time, evidence from meta-analysis and post hoc analysis of large-scale participating trials did not show a significant increase in the benefits of BP <130/80 mm Hg. Since then, new information has emerged from post hoc analysis of the results of large trials in patients at high CV risk. New RCTs and meta-analysis of all available RCTs.
In post hoc analysis of RCTs and registry data, compared with a target SBP between 130 mmHg and 139 mmHg, reducing SBP <130 mmHg was associated with greater stroke risk reductions, particularly in patients with type 2 diabetes. 2. A consistent finding was that reducing SBP <120 mmHg increased the incidence of CV events and death.
Two major RCTs addressing this issue have recently been published, the SPRINT trial and the STEP trial. The first compared 2 different SBP targets (<140 or <120 mmHg) in >9,000 hypertensive patients at high CV risk, but patients with diabetes or previous stroke were excluded. More intensive antihypertensive treatment with target SBP <130 mmHg (mean achieved SBP 121 vs. 136 mmHg in the standard arm) was associated with a 25% reduction in major CV events and a 27% reduction in all-cause death. , although no significant reduction in stroke was observed.
It is worth mentioning that according to the inclusion criteria, the patients in the SPRINT study were not naïve to antihypertensive treatment and were able to tolerate 3 drugs without orthostatic hypotension. It was not representative of the average of the untreated population. The STEP trial had a similar design that compared 2 different SBP targets (<150 or <130 mmHg) in more than 8,000 Chinese hypertensive patients aged 60 to 80 years, excluding those with a history of stroke or hemorrhagic stroke.
During a mean follow-up period of 3.34 years, the most intensive hypotensive treatment that achieved SBP <130 mmHg (mean achieved 127.5 vs. 135.3 mmHg in the standard arm) was associated with a 26% reduction in major CV events and a 28% reduction in death from CV causes. E stroke was also significantly reduced by 33%. Regarding safety and renal outcomes, there were no significant differences between the 2 groups, except for the incidence of orthostatic hypotension, which was higher in the intensive treatment group. These latter 2 studies provide indisputably strong support for the beneficial effects of more intense BP lowering versus intensive therapeutic strategies in higher-risk patients.
The authors conclude that, taking into account the results of these 2 RCTs and those of the meta-analysis, there is agreement with the new recommendations of the guidelines, in achieving the objectives of SBP <130 mmHg, as long as the treatment is well tolerated. In patients of In advanced age, a more cautious SBP target (<140 mmHg) is recommended, although a target <130 mmHg would be better to prevent primary strokes in subjects up to 80 years of age, if there is good tolerance to the treatment and orthostatic hypotension is avoided.
| Recommendations for the management of BP in patients with hemorrhagic stroke | Class | Level |
| Immediate lowering of SBP <220 mmHg is not recommended | III | TO |
| In patients with SBP ≥220 mmHg: acute treatment of BP, with intravenous medications to achieve a BP <180 mmHg. | IIa | b |
| NOTE: Recommendations from the 2018 ESC/ESH hypertension guidelines on BP management in patients in the acute phase of hemorrhagic stroke | ||
| 7. Target blood pressure values during the acute phase of hemorrhagic stroke |
Elevated BP is very common in acute hemorrhagic stroke due to several factors; Elevated SBP is associated with greater hematoma expansion and neurological deterioration, dependency, and death. Compared with stroke, in which consistent J-shaped associations have been found between nadir SBPs of 140 and 150 mmHg and poor outcomes, the main problem with hemorrhagic stroke is high SBP above 150 mmHg. For these patients, SBP between 150 and 220 mmHg, if there are no contraindications to acute BP treatment.
The 2015 AHA/ASA guidelines recommended acute reduction of SBP <140 mmHg as safe and may be effective in improving outcome. For hemorrhagic stroke patients with SBP >220 mmHg, it may be reasonable to consider aggressive BP reduction through continuous intravenous infusion and frequent monitoring. These recommendations were based on the results of 2 studies, INTERACT-2 and the trial by Qureshi et al, and a meta-analysis.
INTERACT-2 is an international open-label phase 3 RCT, with blinded end-point examination (PROBE design), with 2,839 patients with spontaneous intracranial hemorrhage 6 hours after hemorrhagic stroke, with SBP between 150 and 220 mmHg. Patients were randomized to implement intensive BP reduction (target <140 mmHg) or guideline-recommended treatment (BP <180 mmHg) in the first hour.
Intensive treatment did not reduce the primary outcomes of death or major disability at 3 months, but reduction in hematoma expansion improved functional recovery and was safe, with no difference in side effects compared with contemporary control of SBP <180. mmHg, recommended by the guidelines. An additional analysis of the INTERACT-2 data set explored whether different degrees of SBP reduction by treatment during the 7 days after hemorrhagic stroke were associated with the risk of poor outcomes at 90 days, and whether the benefit/harm ratio varied by BP level at presentation.
Greater reductions in SBP (20 and 30 mmHg during the first hour after randomization) were significantly associated with lower risks of poor outcomes compared with the minimum reduction <10 mmHg. On the other hand, optimal recovery from hemorrhagic stroke was observed in hypertensive patients who achieved the greatest reductions in BP (≥20 mmHg) in the first hour and maintained it for 7 days. However, in a subsequent RCT of 1,000 patients with hemorrhagic stroke and SBP <220 mmHg, 500 were assigned to a target SBP of 110 to 139 mmHg (intensive treatment) and the other 500 to a target of 140 to 179 mmHg (standard treatment). .
The aim of the study was to test whether intensive SBP lowering with intravenous nicardipine provides better outcomes than standard treatment. Nicardipine was administered within 4.5 h of symptom onset. Similar to the INTERACT-2 trial, the primary outcome 3 months after randomization was death or major disability. The primary outcome was observed in 38.7% of participants in the intensive treatment group and 37.7% in the standard treatment group.
More intensive BP reduction had no benefit on the similar primary outcome and was associated with more renal adverse events. Regarding patients with hemorrhagic stroke and PAX ≥220 mmHg, Tsivgoulis et al. performed a systematic review and meta-analysis of all RCTs that randomly selected patients with acute hemorrhagic stroke to receive intensive or guideline treatment for BP reduction. After identifying 4 eligible studies that included 3,315 patients, the meta-analysis showed that mortality rates were similar in both groups, although intensive hypotensive treatment showed a tendency to be associated with a lower rate of death or dependence at 3 months, compared to with the prescribed treatment.
Another recent meta-analysis of clinical trial data demonstrated that intensive therapy safely reduced SBP, as well as hematoma expansion, but this reduction did not lead to a better functional outcome. Added to this, a secondary analysis of the ATACH2 trial, in which rapid intensive BP reduction was performed within 2 h of hemorrhagic stroke symptom onset, reduced hematoma expansion and improved functional outcome.
In summary, the authors state that the goal of achieving optimal SBP in patients with acute hemorrhagic stroke remains a matter of debate and, despite evidence that hematoma expansion is reduced in intensively treated patients, this approach did not consistently lead to to better results.
In patients with mild to moderate hemorrhagic stroke and systolic BP between 150 and 220 mmHg, aggressive reduction of SBP to a target of 120-139 mmHg is relatively safe, although it has a potential risk of kidney injury, and could improve functional outcome , and restrict hematoma expansion. Ideally, treatment should begin within 2 h of symptom onset, achieving target systolic BP without problems.
The 2018 ESC/ESH hypertension guidelines stated that BP lowering is not recommended as a routine approach for patients with hemorrhagic stroke and SBP <220 mmHg, and for patients with hemorrhagic stroke and SBP ≥220 mmHg. Careful acute reduction of BP to <180 mmHg should be considered as a therapeutic goal. However, more recent data suggest that a SBP reduction <140 mmHg may be considered in patients with mild to moderate hemorrhagic stroke and baseline SBP between 150 and 220 mmHg.
| 8 . Blood pressure target values during the acute phase of ischemic stroke |
As occurs in hemorrhagic stroke, HTN is a problem in the early phases of acute stroke while SBP elevations >160 mmHg are observed in more than 60% of patients, being associated with cerebral edema and poor outcomes. Theoretically, the reasons for lowering BP in this clinical situation include reducing cerebral edema formation, minimizing the risk of hemorrhagic transformation of infarction, preventing further vascular damage, and decreasing the risk of early recurrent stroke. However, in the majority of patients without any specific medical treatment, there is a spontaneous reduction in SBP of around 10-15 mmHg during the first 24 hours. Therefore, aggressive treatment of all patients with high initial SBP can lead to neurological worsening, resulting from reduced perfusion pressure in ischemic brain areas, worsening neuronal hypoxia.
The pathophysiology of the transient increase in BP in acute stroke is complex and poorly understood. The intervention of several factors has been suggested, some of which are non-specific and unrelated to the cerebral ischemic process, such as psychological stress, while others are specific to stroke, such as stroke subtypes.
The results of trials that studied the link of HTN during the first hours after stroke with short- and long-term clinical outcomes after stroke are particularly conflicting. In fact, HTN during the acute phase can be beneficial since it maintains cerebral blood flow in the ischemic tissue but, on the other hand, it can be harmful since it facilitates cerebral edema and hemorrhagic transformation. Although many studies have addressed the issue of BP management in acute stroke, their interpretation is hampered by important methodological problems that lead to diverse results among the populations investigated. Therefore, the correct management of hypertension in acute stroke remains unclear and the available evidence is insufficient to demonstrate that BP reduction reduces mortality or disability in patients with acute ischemic stroke. However, there are some indications of the possible effectiveness of very early BP lowering. However, reliable data on the influence of BP values on stroke outcomes are lacking, and therefore BP management in the acute phase of stroke remains rather empirical. The current recommendations of all guidelines recognize that they are based on opinion rather than evidence.
| 9 . How is blood pressure managed in the acute phase of ischemic stroke? |
The temporal pattern of BP and the magnitude of BP change in the first 24 h after stroke appears to be much more prognostic than baseline BP alone. Admission BP is often misleading due to transient nonspecific BP fluctuations following an acute critical illness such as acute stroke. Consecutive BP recordings in the acute phase of stroke (e.g., 24-h BP monitoring) may more reliably indicate the factors leading to the acute hypertensive response.
Continuous or intermittent 24-h BP monitoring correlates more closely with target organ damage and major CV events, including edema in acute stroke, than isolated office BP values. Furthermore, compared with individual recordings, 24-h monitoring reduces the pressure response to hospital admission, observer bias, and measurement variability.
BP monitoring allows rapid changes in BP during an acute stroke to be measured more accurately. Therefore, continuous or intermittent BP monitoring, with relative rather than absolute changes, could provide better prognostic information about the course and range of BP fluctuations than casual BP.
Recently, higher BP values derived from 24-h monitoring, as opposed to BP during hospitalization, have been associated with stroke outcome. However, it is still unclear whether this association is causal or not. However, the best option seems to be close monitoring of BP recordings in the first 24 h, although data supporting active BP modulation with antihypertensive therapy during acute stroke are lacking.
In recent years, several randomized clinical trials evaluated the benefits and risks of reducing BP during acute stroke through antihypertensive treatment. However, most trials investigated the effect of interventions in the subacute phase, starting within 24 to 72 hours after stroke onset.
The median time to start BP reduction in 13 controlled trials, with 12,703 participants, was no earlier than 15 hours after stroke onset. In general, trials addressing this issue found no beneficial effects of BP-lowering therapy. The SCAST is a double-blind, placebo-controlled RCT with 2,029 patients with acute stroke (ischemic or hemorrhagic) and SBP ≥140 mm Hg who were included within the first 30 hours of symptom onset. Patients were randomly assigned to candesartan or placebo for 7 days, with the dose increasing from 4 mg on day 1 to 16 mg on days 3 to 7.
The two co-primary endpoints were: 1) a composite endpoint of vascular death, myocardial infarction, or stroke during the first 6 months and, 2) functional outcome at 6 months. After 6 months of follow-up, the risk of the composite vascular endpoint was not different between the treatment groups, and functional outcome analysis suggested a higher risk of poor outcome in the treated group.
No differences were observed between the 2 groups for all prespecified secondary endpoints, including death from any cause, vascular death, stroke and hemorrhagic stroke, myocardial infarction, stroke progression, symptomatic hypotension, and renal failure. .
A secondary analysis of the SCAST trial aimed to investigate the effect of lowering SBP during the first 2 days after stroke on the risk of adverse events and short- and long-term outcome in the entire population, regardless of the assigned treatment. Of note, patients with a marked decrease or increase/no change in SBP had a significantly higher risk of early adverse events compared with that of patients with small decreases.
Patients with an increase or no change in BP and those with a large reduction in SBP >28 mmHg had a significantly increased risk of poor neurological outcome compared to groups with a small (<15 mmHg) or moderate (15-28 mmHg) decrease. mmHg) of the PAAS, adopting a “U”-shaped curve. These results support the ESC/ESH 2018 hypertension guidelines, which recommend avoiding large reductions in BP, and that BP reduction should not be a routine action in the acute phase of stroke except in 2 clinical situations: 1) in patients eligible for intravenous thrombolysis, BP should be cautiously reduced and maintained below 180/105 mmHg, at least for 24 hours after thrombolysis and, 2) in patients with markedly elevated SBP levels and sustained during the first 24 hours, which are not candidates for fibrinolysis. A small/moderate reduction in SBP of around 15% of baseline values may be considered.
The timing of PA intervention could be crucial. There is some evidence for the possible effectiveness of very early BP reduction within 6 h of stroke onset, although this is based on subgroup analysis of a single trial. On the other hand, the results of an observational study suggest that, in patients with acute stroke, BP reduction and the use of hypotensive agents during the first 24 hours could be associated with a lower risk of poor prognosis, regardless of whether they were treated. or not with a recombinant tissue plasminogen activator. Also a subanalysis of the China Antihypertensive Trial i in acute stroke suggests that BP reduction could reduce death, severe disability, and recurrent stroke in Chinese patients who received antihypertensive treatment between 24 and 48 h after stroke onset.
No beneficial effect was found when treatment started within 12 h. Further research is required to establish whether the timing of BP reduction is important in the treatment of patients with acute stroke, and whether it is associated with the presence of live penumbra. In summary, a recent statement by the Group on Hypertension and the Brain of the European Society of Hypertension concluded that BP is highly dynamic and not a static entity in the acute stage, and should ideally be measured continuously during the acute phase. of ischemic stroke.
Currently, there is no convincing data to support routine BP reduction during the first hours or days of stroke, although recent results suggest that early HBP reduction in ischemic stroke may be beneficial in preventing death or dependency. at least in some subgroups. This requires confirmation in additional studies to identify patients most likely to benefit from BP reduction in stroke, and to establish the time window in which treatment is most likely to lead to a favorable response.
| Recommendations for the management of BP in patients with acute ischemic stroke | Class | Level |
| Routine lowering of BP is not recommended in patients with acute stroke. | II | TO |
| In patients eligible for thrombolysis, IV BP should be cautiously reduced and maintained <180/105 mmHg, at least 224 h after thrombolysis. | IIa | b |
| In patients with a marked increase in BP who do not receive fibrinolysis, pharmacological treatment can be given according to clinical criteria, to reduce 15% of the BP[A during the first 24 hours after the onset of symptoms. | IIb | c |
| NOTE: Recommendations from the 2018 ESC/ESH hypertension guidelines on the management of P during the acute phase of ischemic stroke | ||
| 10 . Target blood pressure values for secondary stroke prevention |
Reducing BP after a stroke reduces the risk of recurrence and other vascular events. All guidelines state that stroke survivors, both hypertensive and normotensive, excluding those with symptomatic hypotension, should be treated with antihypertensives and make lifestyle changes. However, there is evidence that low BP can lead to a poor outcome.
The NEMESIS (North East Melbourne Stroke Incidence Study) study, aimed at investigating the relationship between BP and outcome in 5-year stroke survivors, showed that SBP ≤120 mmHg was associated with a 61% increased risk of recurrent stroke , acute myocardial infarction and death compared with patients with a reference SBP category of 131-141 mmHg compared with the reference category, there was no difference in the outcome of patients with SBP 121 to 130 mmHg. This study confirmed that very low SBP <120 mmHg may result in poor prognosis.
The SPS3 trial was a multicenter, randomized clinical trial that aimed to explore the optimal target BP to achieve after stroke. Participants were randomized to 2 different SBP goals: the standard 130-149 mmHg, and the intensive <130 mmHg. Over a follow-up of approximately 3.5 years, older participants achieved SBP levels similar to those of younger participants with a mean of 127 mmHg in the intensive group and 138 mmHg in the standard group.
In younger adults, recurrent stroke was less likely in the intensive group than in the standard treatment group but not in older adults. However, the intensive SBP goal was associated with a significant reduction in vascular death in older participants.
The study clearly showed that intensive treatment significantly reduced the risk of cerebral hemorrhage by 63%, but did not reduce the risk of recurrence of lacunar infarcts. A J-shaped association was found between the BP achieved and the results. The subjects who had the lowest risk were those with SBP between 128 mmHg/67 mmHg and diastolic BP of 65–70 mmHg. Below these levels, the risk of mortality increased.
A systematic review and meta-regression analysis of the association between BP reduction, recurrent stroke and CV events, using data from 42,736 patients from 14 arms of clinical RCT trials on secondary stroke prevention, showed that SBP reduction It was linearly and significantly related to a lower risk of recurrent stroke, myocardial infarction, death from any cause, CVD, and death. Similarly, reduction in diastolic BP was linearly related to lower risk of recurrent stroke and overall mortality. All these results clearly show that strict and aggressive control of BP is essential for the secondary prevention of stroke, and a target BP <130/80 mmHg should be established for all patients between 18 and 80 years of age.
In patients >80 years, the Berlin Initiative Study, an ongoing, prospective cohort study of individuals ≥70 years of age initiated in 2009, showed that of 1,628 patients (mean age 81 years) treated with antihypertensives, 636 exhibited normalized BP (< 140/90 mmHg) and 992 had BP values ≥140/90 mmHg. Compared with non-normalized BP, normalized BP was associated with a higher risk of overall mortality. The risks observed were increased in patients >80 years old and in those with previous CV events but not in patients aged 70-79 years.
Taking current evidence into account, all guidelines recommend a target BP <130/80 mmHg for patients aged 18 to 79 years with a history of stroke or transient ischemic attack and, in older patients, using more cautious BP targets and regimens of more prudent and individualized medications.