The extrapulmonary manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are now widely recognized and have important clinical implications. To our knowledge, the association of SARS-CoV-2 with respiratory muscles has not been studied . This is surprising, since respiratory muscles drive alveolar ventilation and their weakness causes acute respiratory failure.
In critically ill patients undergoing ventilation, respiratory muscle weakness prolongs mechanical ventilation and increases mortality.
The objective of this study was to investigate the association of severe coronavirus disease 2019 (COVID-19) with respiratory muscles in critically ill patients and compare the findings with those obtained from non-COVID-19 critically ill patients.
Methods
Our study focused on the diaphragm , the main breathing muscle. Consecutive diaphragm muscle samples were collected during autopsy from the cadavers of 26 patients who had been critically ill with COVID-19 at 3 academic medical centers in the Netherlands (known as COVID-19 - intensive care unit [ICU]). in April and May 2020.
As a control group , autopsy diaphragm samples were collected from the corpses of 8 patients who had been critically ill without COVID-19 (referred to as control-ICU).
Samples from the left midcostal diaphragm were used for the analyses.
This study was approved by the medical ethics committee at Amsterdam UMC, and written informed consent was provided by the next of kin of the deceased. Data were analyzed with SPSS, version 22 (IBM) and visualized with GraphPad Prism, version 7.0 (GraphPad). Statistical significance was set at p < 0.05.
Results
The median age of COVID-19 - ICU patients was 71 years (interquartile range, 61-74 years) and 21 (81%) were men.
Twenty-four patients (92.3%) received invasive mechanical ventilation for a median of 12 days (interquartile range, 6-25 days). The number of days receiving invasive mechanical ventilation and length of ICU stay were comparable between COVID-19 - ICU and control - ICU patients.
COVID-19: ICU patients had a higher body mass index (calculated as weight in kilograms divided by height in meters squared) and were less likely to be treated with steroids.
No patient in either group had preexisting neuromuscular disease.
We report angiotensin-converting enzyme 2 (ACE-2) in the diaphragm of patients with COVID-19 - ICU and control - ICU. ACE-2 is predominantly localized to the myofiber membrane, providing an entry point for SARS-CoV-2 to infect diaphragm myofibers. Evidence of SARS-CoV-2 viral RNA was found in the diaphragm in 4 patients (15.4%).
Further analyses, for which we applied RNA in situ hybridization , indicated that the viral RNA was localized within the myofibers of the diaphragm. RNA sequencing analyzes showed that 315 genes were upregulated and 281 downregulated in the diaphragm of COVID-19 ICU patients compared to control ICU patients.
Further analyzes of all upregulated and downregulated genes revealed activation of fibrosis (fibroblast growth factor signaling) pathways.
Consistent with these findings, epimysial and perimysial fibrosis was more than 2-fold higher in the diaphragms of COVID-19 ICU patients compared to control ICU patients.
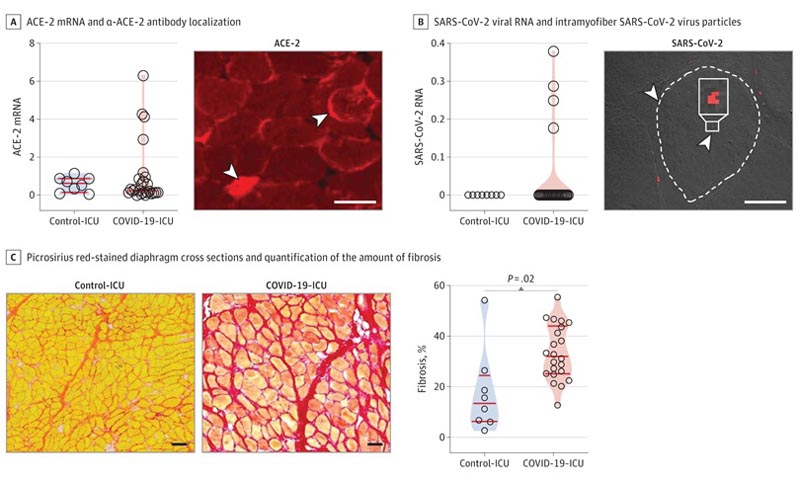
A, left panel: ACE-2 mRNA in diaphragm samples determined by quantitative polymerase chain reaction (qPCR) and normalized to the housekeeping gene TBP. Right panel: localization of α-ACE-2 antibody with fluorescein microscopy in cross sections of the diaphragm; arrowheads show cytosolic and membrane localization (bar = 50 μm). B, Left panel: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA, determined by qPCR and normalized to the TBP housekeeping gene, is detected in the diaphragm of 4 coronavirus 2019 (COVID-19) - unit intensive care (ICU) patients (patients 7, 9, 33 and 36). Right panel: in situ hybridization using RNAscope in patient #7 shows intramifiber SARS-CoV-2 virus particles (red dots, indicated with arrowheads); a myofiber edge is highlighted with a dashed line (bar = 30 μm). C, left panels: representative images of cross sections of the diaphragm stained picrosirius red to highlight fibrosis; patients #22 and 3 are shown (bar = 100 μm). Right panel: quantification of the amount of fibrosis.
Discussion
In this study, we provide unique evidence of ACE-2 expression in the human diaphragm and SARS-CoV-2 viral infiltration in the diaphragm of a subset of COVID-19 patients - ICU.
In patients with COVID-19 - ICU, we report increased expression of genes involved in fibrosis and histological evidence for the development of fibrosis in the diaphragm. This myopathic phenotype was clearly different from that of control-ICU patients, with comparable duration of mechanical ventilation and ICU stay.
It remains to be established whether diaphragmatic myopathy is a direct effect of SARS-CoV-2. Only 3 patients in the control-ICU group (37.5%) had viral lung disease and the association of viral pneumonia with the diaphragm muscles is unknown.
| We hypothesize that severe diaphragmatic myopathy associated with COVID-19, as described in this study, may lead to diaphragm weakness and could contribute to ventilator weaning failure, persistent dyspnea, and fatigue in patients with COVID-19 who They survive their stay in the ICU. |