Meaning COVID-19 is currently a major threat to global health. However, there are no specific antiviral agents available for its treatment. In this work, we explore the feasibility of convalescent plasma (CP) transfusion to rescue critically ill patients. The results of 10 severe adult cases showed that one dose (200 ml) of PC was well tolerated and could significantly increase or maintain neutralizing antibodies at a high level, leading to disappearance of viremia within 7 days. Meanwhile, clinical symptoms and paraclinical criteria improved rapidly within 3 days. Radiological examination showed varying degrees of absorption of lung lesions in 7 days. These results indicate that PC may serve as a promising rescue option for severe COVID-19, while randomized trial is warranted. |
Since December 2019, a pneumonia associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), emerged in Wuhan, China.
The epidemic spread rapidly around the world within 3 months and was characterized as a pandemic by the WHO on March 11, 2020. As of March 12, 2020, a total of 80,980 confirmed cases and 3,173 deaths had been reported in China. Meanwhile, a total of 44,377 confirmed cases and 1,446 deaths were reported in 108 other countries or regions.
Currently, there are no specific antiviral agents approved that target the new virus, while some drugs are still under investigation, including remdesivir and lopinavir/ritonavir. Although remdesivir was reported to have a possible antiviral effect in a COVID-19 patient in the United States, randomized controlled trials of this drug are underway to determine its safety and effectiveness.
Furthermore, corticosteroid treatment for COVID-19 lung injury remains controversial, due to delayed clearance of viral infection and complications. Since effective vaccine and specific antiviral drugs are not available, it is an urgent need to seek an alternative strategy for treatment with COVID-19, especially among severe patients.
Convalescent plasma (CP) therapy , a classic adaptive immunotherapy, has been applied to the prevention and treatment of many infectious diseases for more than a century. Over the past two decades, CP therapy has been successfully used in the treatment of SARS, MERS, and the 2009 H1N1 pandemic with satisfactory efficacy and safety.
A meta-analysis of 32 studies of SARS coronavirus infection and severe influenza showed a statistically significant reduction in the pooled odds of mortality after CP therapy, compared with placebo or no therapy (odds ratio, 0.25; 95% confidence interval). %, 0.14–0.45). However, CP therapy failed to significantly improve survival in Ebola virus disease , probably due to the absence of neutralizing antibody titration data for stratified analysis.
Since the virological and clinical features share similarities between SARS, Middle East Respiratory Syndrome (MERS), and COVID-19, CP therapy could be a promising treatment option for the rescue of COVID-19 (16). Patients who have recovered from COVID-19 with a highly neutralizing antibody titer may be a valuable source of PC donors.
However, the potential clinical benefit and risk of convalescent blood products in COVID-19 remains uncertain. Therefore, we conducted this pilot study in three participating hospitals to explore the feasibility of CP treatment in 10 severely ill COVID-19 patients.
Methods
A transfusion of 200 ml of convalescent plasma (CP) derived from recently recovered donors with neutralizing antibody titers greater than 1:640 was transfused to patients as an addition to maximal supportive care and antiviral agents.
- The primary objective was the safety of CP transfusion.
- The second endpoints were improvement in clinical symptoms and laboratory parameters within 3 days after PC transfusion.
The median time from disease onset to PC transfusion was 16.5 days. After PC transfusion, the level of neutralizing antibodies increased rapidly to 1:640 in five cases, while that of the other four cases remained at a high level (1:640).
Results
The clinical symptoms improved significantly along with the increase in oxyhemoglobin saturation within 3 days.
Several parameters tended to improve compared to pretransfusion, including increased lymphocyte counts (0.65 × 109/L vs. 0.76 × 109/L) and decreased C-reactive protein (55.98 mg /L vs. 18.13 mg/L).
Radiological examinations showed varying degrees of absorption of lung lesions within 7 days. Viral load was undetectable after transfusion in seven patients who had prior viremia.
No serious adverse effects were observed.
This study showed that CP therapy was well tolerated and could improve clinical outcomes by neutralizing viremia in severe cases of COVID-19. The optimal dose and time point, as well as the clinical benefit of CP therapy, need further investigation in larger, well-controlled trials.
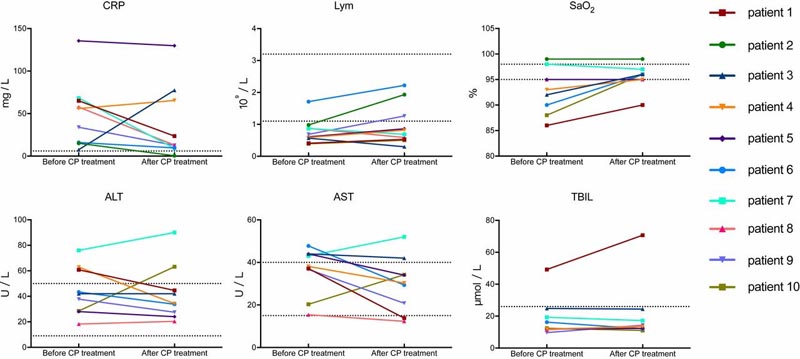 Dynamic changes of laboratory parameters in all patients. The dashed horizontal line represents the range of reference values. SaO2, oxyhemoglobin saturation; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Lym, lymphocytes.
Dynamic changes of laboratory parameters in all patients. The dashed horizontal line represents the range of reference values. SaO2, oxyhemoglobin saturation; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Lym, lymphocytes.
Discussion
Our study explores the feasibility of CP therapy in COVID-19. All patients enrolled in severe COVID-19 achieved primary and secondary outcomes. A CP transfusion dose of 200 ml was well tolerated, while clinical symptoms improved significantly with increasing oxyhemoglobin saturation within 3 days, accompanied by rapid neutralization of viremia.
Severe pneumonia caused by human coronavirus was characterized by rapid viral replication, massive inflammatory cell infiltration, and elevated proinflammatory cytokines or even cytokine storm in the alveoli of the lungs, resulting in acute lung injury and acute respiratory distress syndrome. (ARDS).
Recent studies on COVID-19 demonstrated that lymphocyte counts in the peripheral blood were markedly decreased and cytokine levels in the plasma of patients requiring intensive care unit (ICU) support, including IL-6, IL-10 , TNF, and granulocyte-macrophage colony-stimulating factor, were significantly higher than in those who did not require ICU conditions.
Convalescent plasma (CP), obtained from recovered COVID-19 patients who had established humoral immunity against the virus, contains a large amount of neutralizing antibodies capable of neutralizing SARS-CoV-2 and eradicating the pathogen from the blood circulation and lung tissues.
In the present study, all investigated patients achieved serum SARS-CoV-2 RNA negativity after PC transfusion accompanied by increased oxygen saturation and lymphocyte counts, and improvement of liver function. and PCR.
The results suggest that the inflammation and overreaction of the immune system were alleviated by the antibodies contained in PC.
The case fatality rates (CFR) in the present study were 0% (0/10), which was comparable to the CFRs in SARS, which ranged from 0% (0/10) to 12.5% (10/80) in four non-comparative studies with CP treatment (9, 20 ⇓ – 22). Based on our preliminary results, PC therapy may be an easily accessible, promising, and safe rescue option for patients with severe COVID-19. However, it is worth mentioning that the absorption of lung lesions was often inferior to the improvement of clinical symptoms, as shown in patients 9 and 10 in this trial.
The first key factor associated with CP therapy is the neutralizing antibody titer.
A small sample study in MERS-CoV infection showed that the neutralizing antibody titer must exceed 1:80 to achieve effective CP therapy. Finding eligible donors who have high levels of neutralizing antibodies is a prerequisite.
Cao et al. showed that the level of specific neutralizing antibodies against SARS-CoV gradually decreased 4 months after the disease process, reaching undetectable levels in 25.6% (IgG) and 16.1% (neutralizing antibodies) of patients at 36 months after the disease state. the illness.
A study of MERS-CoV-infected patients and exposed healthcare workers showed that the prevalence of MERS-CoV IgG seroreactivity was very low (2.7%), and the antibody titer decreased rapidly within 3 months. These studies suggested that neutralizing antibodies represented a short-lived humoral immune response , and that plasma from recently recovered patients should be more effective.
In the present study, recently recovered COVID-19 patients, who were infected by SARS-CoV-2 with a neutralizing antibody titer above 1:640 and recruited from local hospitals, should be considered suitable donors. The median age of donors was lower than that of recipients (42.0 y vs. 52.5 y). Among the nine cases investigated, the neutralizing antibody titers of five patients increased to 1:640 within 2 days, while four patients maintained the same level. Antibody titers in CP in COVID-19 therefore appear higher than those used in the treatment of the MERS patient (1:80).
The second key factor associated with effectiveness is the time point of treatment.
Better treatment outcome was observed among SARS patients who received CP before 14 dpoi (58.3% vs. 15.6%; P < 0.01), highlighting the importance of timely rescue therapy. The mean time from disease onset to PC transfusion was 16.5 days.
Consistent with previous research, the three patients who received plasma transfusions administered before 14 dpoi (patients 1, 2, and 9) in our study showed a rapid increase in lymphocyte counts and a decrease in CRP, with notable absorption of lung lesions on CT.
In particular, patients who received PC transfusion after 14 dpoi showed much less significant improvement, such as patient 10. However, the dynamics of SARS-CoV-2 viremia were unclear, so the point of Optimal transfusion time should be determined in the future.
In the present study, no serious adverse effects were observed.
One of the risks of plasma transfusion is the transmission of the possible pathogen. Methylene blue photochemistry was applied in this study to inactivate possible residual virus and maintain the activity of neutralizing antibodies as much as possible, a method known to be much better than ultraviolet (UV) C light (25). . No specific virus was detected before transfusion.
A transfusion-related acute lung injury was reported in a woman with Ebola virus disease who received PC therapy. Although rare in the general population receiving plasma transfusion, this specific adverse reaction is worth noting, especially among critically ill patients who experience significant lung injury.
Another rare risk worth mentioning during PC therapy is antibody-dependent enhancement of infection, which occurs at subneutralizing concentrations, which could suppress innate antiviral systems and thus could allow logarithmic intracellular growth of the virus. Special improvement of infection could also be found in SARS-CoV infection in vitro. No lung injury and improvement of infection was observed in our patients, probably due to high levels of neutralizing antibodies, timely transfusion, and appropriate plasma volume.
There were some limitations to the present study.
- First, except for PC transfusion, patients received other standard care. All patients received antiviral treatment despite uncertainty about the effectiveness of the medications used. As a result, the possibility that these antiviral agents may contribute to patients’ recovery or synergize with the therapeutic effect of PC cannot be ruled out. Additionally, some patients received glucocorticoid therapy , which could interfere with the immune response and delay virus clearance.
- Second, the median time from symptom onset to PC transfusion was 16.5 days (IQR, 11.0 days to 19.3 days). Although the kinetics of viremia during the natural history remain unclear, the relationship between SARS-CoV-2 RNA depletion and CP therapy, as well as the optimal concentration of neutralizing antibodies and treatment schedule, should be clarified. even more.
- Third, dynamic changes of cytokines during treatment were not investigated. However, the preliminary results of this trial appear promising, warranting a randomized controlled clinical trial in a larger patient cohort.
In conclusion , this pilot study on PC therapy shows potential therapeutic effect and low risk in treating patients with severe COVID-19. A dose of PC with a high concentration of neutralizing antibodies can rapidly reduce viral load and tends to improve clinical outcomes. The optimal dose and treatment time point, as well as the definitive clinical benefits of CP therapy, should be further investigated in randomized clinical studies. |